Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment
Funding information: Deutsche Forschungsgemeinschaft, Grant/Award Number: KFO 286
Abstract
Disease overview
Chronic lymphocytic leukemia (CLL) is the commonest leukemia in western countries. The disease typically occurs in elderly patients and has a highly variable clinical course. Leukemic transformation is initiated by specific genomic alterations that impair apoptosis of clonal B-cells.
Diagnosis
The diagnosis is established by blood counts, blood smears, and immunophenotyping of circulating B-lymphocytes, which identify a clonal B-cell population carrying the CD5 antigen, as well as typical B-cell markers.
Prognosis
The two similar clinical staging systems, Rai and Binet, create prognostic information by using results of physical examination and blood counts. Various biological and genetic markers also have prognostic value. Deletions of the short arm of chromosome 17 (del [17p]) and/or mutations of the TP53 gene, predict resistance to chemoimmunotherapy and a shorter time to progression, with most targeted therapies. A comprehensive, international prognostic score (CLL-IPI) integrates genetic, biological and clinical variables to identify distinct risk groups of CLL patients.
Therapy
Only patients with active or symptomatic disease, or with advanced Binet or Rai stages require therapy. When treatment is indicated, several options exist for most CLL patients: a combination of venetoclax with obinutuzumab, ibrutinib monotherapy, or chemoimmunotherapy. For physically fit patients younger than 65 (in particular when presenting with a mutated IGVH gene), chemoimmunotherapy with fludarabine, cyclophosphamide and rituximab remains a standard therapy, since it may have curative potential. At relapse, the initial treatment may be repeated, if the treatment-free interval exceeds 3 years. If the disease relapses earlier, therapy should be changed using an alternative regimen. Patients with a del (17p) or TP53 mutation are a different, high-risk category and should be treated with targeted agents. An allogeneic SCT may be considered in relapsing patients with TP53 mutations or del (17p), or patients that are refractory to inhibitor therapy.
Future Challenges
Targeted agents (ibrutinib, idelalisib, venetoclax, obinutuzumab) will be increasingly used in combination to allow for short, but potentially definitive therapies of CLL. It remains to be proven that they generate a superior outcome when compared to monotherapies with inhibitors of Bruton tyrosine kinase, which can also yield long-lasting remissions. Moreover, the optimal sequencing of drug combinations is unknown. Therefore, CLL patients should be treated in clinical trials whenever possible.
1 INTRODUCTION AND DISEASE OVERVIEW
With an age-adjusted incidence of 4.1/100 000 inhabitants in the United States, chronic lymphocytic leukemia (CLL) is the most common type of leukemia in western countries.1 More than 15 000 newly diagnosed cases and approximately 4500 deaths are currently estimated.1, 2 The median age at diagnosis lies at 72 years. More male than female patients (1.7, 1) are affected.3-5 As the incidence rate rises with age, the prevalence and mortality of CLL are likely to increase further due to the demographic changes in society in the forthcoming decades. Moreover, the proportion of younger patients with early stage CLL and minimal symptoms seems to increase due to more frequent blood testing.6
Chronic lymphocytic leukemia is characterized by the clonal proliferation and accumulation of mature, typically CD5-positive B-cells within the blood, bone marrow, lymph nodes and spleen.7 Very recently, it has been reported that in CLL the capacity to generate clonal B cells might be acquired at the hematopoietic stem cell (HSC) stage,8 suggesting that the primary leukemogenic event in CLL might involve multipotent, self-renewing HSCs. The process of leukemogenic transformation is increasingly understood. A recent, comprehensive description of the genomic landscape of CLL in large cohorts shows that often the disease may be initiated by the loss or addition of large amounts of chromosomal material (eg, deletion 13q, deletion 11q, trisomy 12), followed later by additional mutations that may render the leukemia more aggressive.9
Deletions on the long arm of chromosome 13, specifically involving band 13q14 (del[13q14]) represent the single most frequently observed cytogenetic aberration in CLL, occurring in approximately 55% of all cases. An isolated del(13q14) is characterized by a benign course of the disease. The miRNAs, miR-15a and 16-1, were recently identified to be located in the critical region of del(13q14).10 The pathophysiologic role of these miRNAs is further underscored by the phenotype of genetically engineered mice carrying a targeted deletion of the mir-15a/16-1 locus, in combination with a deletion of the non-coding RNA gene DLEU2. These animals develop a monoclonal B-cell lymphocytosis-like disorder, CLL and lymphoma, suggesting that the miRNAs 15a and 16-1 indeed play a role in CLL leukemogenic.11
Deletions of the long arm of chromosome 11 (del[11q]) can be found in approximately 25% of chemotherapy-naïve patients with advanced disease stages, and 10% of patients with early stage disease.12, 13 These deletions frequently encompass band 11q23 harboring the gene ATM, which encodes for the proximal DNA damage response kinase ATM. In addition, patients carrying a del(11q) clone typically show a bulky lymphadenopathy, rapid progression, and reduced overall survival.14 Interestingly, some of the poor prognostic features of del(11q) seem to be overcome by the use of chemoimmunotherapy.15
Trisomy 12 is observed in 10% to 20% of CLL patients. However, the genes involved in the pathogenesis of CLL carrying a trisomy 12 are largely unknown. Furthermore, the prognostic relevance of trisomy 12 remains a matter of debate.16
Deletions of the short arm of chromosome 17 (del[17p]) are found in 5% to 8% of chemotherapy-naïve patients. These deletions almost always include band 17p13, where the prominent tumor suppressor gene TP53 is located. The CLL patients carrying a del(17p) clone show marked resistance against genotoxic chemotherapies that cannot be overcome by the addition of anti-CD20 antibodies in the context of state of the art chemo-immunotherapy.15 Mutations of TP53 are found in 4% to 37% of patients with CLL, and have been associated with very poor prognosis in a number of studies.17 Among cases with confirmed del(17p), the majority shows mutations in the remaining TP53 allele (>80%). In cases without del(17p), TP53 mutations are much rarer, but have a similarly detrimental effect on chemotherapy response and overall survival.16 The TP53 mutations are also associated with higher genomic complexity in CLL, indicating that a crippled DDR promotes a “mutator phenotype” in CLL.16
Recent work using whole exome sequencing in CLL has led to the characterization of the genomic landscape of this disease. In addition to the above described chromosomal aberrations, a total number of 44 recurrently mutated genes and 11 recurrent somatic copy number variations has been identified.9 These include the genes NOTCH1, MYD88, TP53, ATM, SF3B1, FBXW7, POT1, CHD2, RPS15, IKZF3, ZNF292, ZMYM3, ARID1A and PTPN11.9, 13, 18, 19 These analyses collectively identify RNA processing and export, MYC activity, and MAPK signaling as central pathways involved in CLL.9 Proteins critically involved in DNA damage signaling and DNA repair are also involved.20 Intriguingly, both del(17p) and del(11q), as well as inactivating somatic mutations in TP53 and ATM are enriched in patients with secondary resistance to DNA damaging chemotherapy.13, 18 In addition, mutations in an enhancer located on chromosome 9p13 may reduce the expression of the B-cell-specific transcription factor PAX5.19
Survival of CLL cells strictly depends on a permissive microenvironment composed of cellular components like macrophages, T cells, or stromal follicular dendritic cells.21-23 They provide stimuli for activation of crucial survival and pro-proliferative signaling pathways in transformed cells. This microenvironment produces various essential proteins (chemokines, cytokines, and angiogenic factors) that interact with leukemic cells via appropriate surface receptors or adhesion molecules to support the survival of CLL cells.23-25 Interestingly, some of the new inhibitors (ibrutinib, idelalisib) also seem to exert their effects by targeting key pathways of microenvironmental cells in CLL patients.26-30
As a consequence of these important advances the management of this leukemia continues to undergo highly relevant improvements that have started approximately 30 years ago.31, 32 Several new drugs have been approved during the last three decades. Chemoimmunotherapies such as fludarabine, cyclophosphamide and rituximab or chlorambucil with obinutuzumab have shown to improve overall survival when used as therapy for CLL patients. More recently, specific inhibitors interrupting important pathways for CLL cell survival have been approved (ibrutinib, idelalisib, venetoclax). Over the past 2 years, these inhibitors have started to replace chemoimmunotherapy in first and second line indications. This updated review integrates the latest innovations in CLL therapy as well as diagnostic tools into an algorithm to guide the diagnostic and therapeutic decisions in daily practice.
2 DIAGNOSIS
This section uses the latest version of iwCLL guidelines33 (with minor modifications); these guidelines give clear recommendations on how to establish the diagnosis of CLL. In most cases the diagnosis of CLL is established by blood counts, differential counts, a blood smear, and immunophenotyping. The World Health Organization (WHO) classification of hematopoietic neoplasias describes CLL as leukemic, lymphocytic lymphoma, being only distinguishable from SLL (small lymphocytic lymphoma) by its leukemic appearance.34 CLL is always a disease of neoplastic B-cells, while the entity formerly described as T-CLL has been named T-cell prolymphocytic leukemia (T-PLL).35
The diagnosis of CLL requires the presence of ≥5000 B-lymphocytes/μL in the peripheral blood for the duration of at least 3 months. The clonality of the circulating B-lymphocytes needs to be confirmed by flow cytometry. The leukemia cells found in the blood smear are characteristically small, mature lymphocytes with a narrow border of cytoplasm and a dense nucleus lacking discernible nucleoli and having partially aggregated chromatin. These cells may be found admixed with larger or atypical cells, cleaved cells or prolymphocytes, which may comprise up to 55% of the blood lymphocytes.36 Finding prolymphocytes in excess of this percentage would favor a diagnosis of prolymphocytic leukemia (B-cell PLL). Gumprecht nuclear shadows, or smudge cells, found as cell debris, are other characteristic morphologic features found in CLL.
Monoclonal B lymphocytosis.33 In the absence of lymphadenopathy or organomegaly (as defined by physical examination or CT scans), cytopenias, or disease-related symptoms, the presence of fewer than 5000 B-lymphocytes per μL blood is defined as “monoclonal B-lymphocytosis” (MBL).37 The presence of a cytopenia caused by a typical marrow infiltrate defines the diagnosis of CLL regardless of the number of peripheral blood B-lymphocytes or of the lymph node involvement. Monoclonal B-lymphocytosis seems to progress to frank CLL at a rate of 1% to 2% per year.37
The definition of SLL requires the presence of lymphadenopathy and the absence of cytopenias caused by a clonal marrow infiltrate. Moreover, the number of B-lymphocytes in the peripheral blood should not exceed 5000/μL. In SLL, the diagnosis should be confirmed by histopathological evaluation of a lymph node biopsy whenever possible.
Immunophenotyping.33 In CLL cells co-express the surface antigen CD5 with the B-cell antigens CD19, CD20, and CD23. The levels of surface immunoglobulin, CD20, and CD79b are characteristically low compared to those found on normal B cells.38-40 Each clone of leukemia cells is restricted to expression of either kappa or lambda immunoglobulin light chains.38 It should be noted that the expression of CD5 can also be observed in other lymphoid malignancies, such as mantle cell lymphoma.41 A recent, large harmonization effort has confirmed that a panel of CD19, CD5, CD20, CD23, kappa and lambda is usually sufficient to establish the diagnosis.42 In borderline cases, markers such as CD43, CD79b, CD81, CD200, CD10, or ROR1 may help to refine the diagnosis.42
3 RISK STRATIFICATION, STAGING, AND INDICATION FOR TREATMENT
Two widely accepted clinical staging systems co-exist, named after the first authors of the original publications, Rai43 and Binet.44 The Rai classification was later modified to reduce the number of prognostic groups from five to three.45 Both systems describe three major prognostic groups with discrete clinical outcomes. These two staging systems are simple, inexpensive, and rely on a physical examination and standard laboratory tests. They do not require ultrasound, computed tomography, or magnetic resonance imaging.
The modified Rai staging system defines low-risk disease as patients who have lymphocytosis with leukemia cells in the blood and/or marrow (lymphoid cells >30%) (former Rai stage 0). Patients with lymphocytosis, enlarged nodes in any site, and splenomegaly and/or hepatomegaly (lymph nodes being palpable or not) are defined as having intermediate risk disease (formerly considered Rai stage I or stage II). High-risk disease includes patients with disease-related anemia (as defined by a hemoglobin [Hb] level less than 11 g/dL) (formerly stage III) or thrombocytopenia (as defined by a platelet count of less than 100 × 109/L) (formerly stage IV).
The Binet staging system is based on the number of involved areas, as defined by the presence of enlarged lymph nodes of greater than 1 cm in diameter or organomegaly, and on whether there is anemia or thrombocytopenia. The areas of involvement considered are (a) head and neck, including the Waldeyer ring (this counts as one area, even if more than one group of nodes is enlarged). (b) axillae (involvement of both axillae counts as one area). (c) Groins, including superficial femoral (involvement of both groins counts as one area). (d) Palpable spleen. (e) Palpable liver (clinically enlarged). The Binet staging system defines stage A as Hb ≥10 g/dL and platelets ≥100 × 109/L and up to two of the above involved; stage B as Hb ≥10 g/dL and platelets ≥100 × 109/L and organomegaly greater than that defined for stage A (ie, three or more areas of nodal or organ enlargement); and stage C as Hb of less than 10 g/dL and/or a platelet count of less than 100 × 109/L.
Due to the recent progress in CLL therapy, the two clinical staging systems have become insufficient to distinguish three or more prognostic subgroups.46 Moreover, the last three decades have generated a plethora of potential markers that provide prognostic information independent of the clinical stage.47 In particular, many of the above defined genetic and chromosomal aberrations have shown prognostic value. To reduce the overwhelming and redundant prognostic information to a few clinically relevant, essential prognostic parameters, comprehensive prognostic scores have been constructed that combine clinical, biological and genetic information.46, 48-50 The currently most relevant prognostic score is the CLL International Prognostic Index (CLL-IPI).51 It uses a weighted grading of five independent prognostic factors: TP53 deletion and/or mutation (collectively called TP53 dysfunction), IGHV mutational status, serum β2-microglobulin, clinical stage, and age. The CLL-IPI separates four groups with different OS at 5 years (see Table 1). The prognostic value of the CLL-IPI will need to be revisited when trials with targeted agents and a longer follow-up will become available.
| CLL-IPI category | OS at 5 years | Potential clinical consequence |
|---|---|---|
| Low-risk | 93.2% | Do not treat |
| Intermediate-risk | 79.3% | Do not treat except if the disease is really symptomatic |
| High-risk | 63.3% | Treatment indicated except if the disease is asymptomatic |
| Very high-risk | 23.3% | If you need to treat do not use chemotherapy but rather novel agents or treatment in clinical trials. |
One very important value of the CLL-IPI also lies in the fact that it identifies - more accurately than the clinical staging - CLL patients without need of therapy. Patients with a low risk CLL-IPI (0-1) and asymptomatic disease do not require treatment. Several studies have shown that treating patients with early stage disease does not result in a survival benefit.52-55 Therefore, an early-intervention therapy with anti-leukemia drugs, including kinase inhibitors or BCL2 antagonists, alone or in combination with monoclonal antibodies, currently is not indicated.
Concise definitions for the initiation of therapy have been proposed by the iwCLL guidelines.33 The decision for initiating treatment depends the presence of active/symptomatic disease. Asymptomatic patients with early-stage disease (Rai 0, Binet A), should be monitored without therapy unless they have evidence of rapid disease progression. So far, studies on early stage disease were unable to show a benefit of early therapeutic interventions.52-55
- Evidence of progressive marrow failure as manifested by the development of, or worsening of, anemia and/or thrombocytopenia. Cut-off levels of Hb < 10 g/dL or platelet counts of <100 000/μL are generally regarded as indication for treatment. However, it should be pointed out that in some patients platelet counts of <100 000/μL may remain stable over a long-period of time; this situation does not always require therapeutic intervention.
- Massive (ie, ≥6 cm below the left costal margin) or progressive or symptomatic splenomegaly.
- Massive nodes (ie, ≥10 cm in longest diameter) or progressive or symptomatic lymphadenopathy.
- Progressive lymphocytosis with an increase of ≥50% over a two-month period, or lymphocyte doubling time (LDT) of less than 6 months. The LDT can be obtained by linear regression extrapolation of absolute lymphocyte counts (ALC) obtained at intervals of 2 weeks over an observation period of 2 to 3 months. Patients with initial blood lymphocyte counts of <30 000/μL may require a longer observation period to determine the LDT. Factors contributing to lymphocytosis other than CLL (eg, infections, steroid administration) should be excluded.
- Autoimmune complications including anemia or thrombocytopenia poorly responsive to corticosteroids.
- Symptomatic or functional extranodal involvement (eg, skin, kidney, lung, spine).
-
Disease-related symptoms as defined by any of the following:
- Unintentional weight loss ≥10% within the previous 6 months.
- Significant fatigue (ie, ECOG PS 2 or worse; cannot work or unable to perform usual activities).
- Fevers ≥100.5°F or 38.0 °C for 2 or more weeks without evidence of infection.
- Night sweats for ≥1 month without evidence of infection.
Hypogammaglobinemia, or monoclonal or oligoclonal paraproteinemia does not by itself constitute a basis for initiating therapy. However, it is recommended to assess the change in these protein abnormalities, if patients are treated. Also, patients with CLL may present with a markedly elevated leukocyte count; however, leukostasis rarely occurs in patients with CLL. Therefore, the absolute lymphocyte count should not be used as the sole indicator for treatment.
4 RESPONSE ASSESSMENT
The iwCLL guidelines give a detailed description of the assessment of the treatment response. A detailed overview of these response criteria is beyond the scope of this manuscript. In essence the following response categories can be separated33: complete remission, partial remission, stable disease and progression, as well as refractory disease. In addition, the assessment of minimal residual disease (MRD) is an additional and increasingly important category of response assessment, resulting in four different response categories (Figure 1).
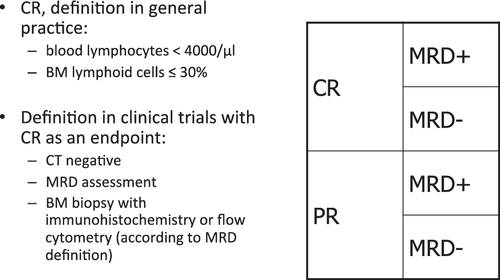
4.1 Eradicating MRD
The use of sensitive multicolor flow cytometry, PCR, or next-generation sequencing can detect minimal residual disease (MRD) in many patients who achieved a complete clinical response. Prospective clinical trials have provided substantial evidence that therapies that are able to eradicate MRD usually result in an improved long-term clinical outcome.56-61 The value of MRD assessments has been compared to the evaluation of clinical response in CLL, in 554 patients treated in two randomized trials of the German CLL Study Group (CLL8 and CLL10).58 Patients with MRD-negative complete remission (CR), MRD-negative PR, MRD-positive CR, and MRD-positive PR, experienced a median PFS of 61, 54, 35, and 21 months, respectively. Interestingly, PFS did not differ significantly between MRD-negative CR and MRD-negative PR. In contrast to residual lymphadenopathy, persisting splenomegaly did not impact outcome in patients with MRD-negative PR. In a retrospective, monocentric analysis, 536 patients were analyzed who achieved at least a partial response (PR) with various therapies between 1996 and 2007, and received a bone marrow MRD assessment at the end of treatment.62 Minimal residual disease negativity correlated with both progression-free survival (PFS) and overall survival (OS) independent of the type and line of treatment, as well as known prognostic factors including adverse cytogenetics. The greatest impact of achieving MRD negativity was seen in patients receiving frontline treatment, with 10-year PFS of 65% vs 10%, and 10-year OS of 70% vs 30%, for MRD-negative vs MRD-positive patients, respectively.
The techniques for assessing MRD have undergone a critical evaluation and have become well standardized.63, 64 Six-color flow cytometry (MRD flow), allele-specific oligonucleotide PCR, or high-throughput sequencing using the ClonoSEQ assay are reliably sensitive down to a level of less than one CLL cell in 10 000 leukocytes.64 Refinement and harmonization of these technologies has established that a typical flow cytometry-based assay comprises a core panel of six markers (ie, CD19, CD20, CD5, CD43, CD79b and CD81).64 As such, patients will be defined as having undetectable MRD (MRD-neg) remission if they have blood or marrow with less than one CLL cell per 10 000 leukocytes. The blood generally can be used for making this assessment, as the marrow will have detectable CLL when it is also found in the peripheral blood. However, there are therapies that preferentially clear the blood but not the marrow (such as monoclonal antibodies). Therefore, it may be important to confirm that the marrow aspirate also is MRD-neg when the blood is found to be MRD-neg. Clinical trials aimed at maximizing the depth of remissions should include at least one test to assess for MRD, because the lack of leukemia persistence using these sensitive tests has a strong, positive prognostic impact. The report should be clear as to whether blood and/or marrow have been assessed and should report the proportion of MRD-neg patients on an intent-to-treat basis, using the total number of patients in that treatment arm as the denominator (not those assessed or those who responded to treatment).
Collectively, there is overwhelming evidence to suggest that MRD quantification allows for improved PFS prediction in both patients who achieve a PR and CR, supporting its application in all responders. Although evaluation of MRD is still not generally recommended for routine clinical practice,33 I anticipate that MRD assessment will be the key variable with regard to the decision to halt therapies with novel inhibitors.32
5 TREATMENT OF CLL
5.1 Active agents in CLL and their use as monotherapy
5.1.1 Cytostatic agents
Monotherapy with alkylating agents has served as initial, front-line therapy for CLL, and chlorambucil was the therapeutic “gold standard” for several decades.55 The advantages of chlorambucil are its low toxicity, low cost and convenience as an oral drug; the major disadvantages are its low to non-existent CR rate and some side effects that occur after extended use (prolonged cytopenia, myelodysplasia and secondary acute leukemia). Today, chlorambucil monotherapy may be used as an inexpensive option to achieve palliation in elderly or unfit patients.
Three purine analogues are used in CLL: fludarabine, pentostatin, and cladribine (2-CdA). Fludarabine remains by far the best-studied compound of the three in CLL. Fludarabine monotherapy produces superior overall response (OR) rates compared with other treatment regimens containing alkylating agents or corticosteroids.65-67 Fludarabine induced more remissions and more complete remissions (CR) (7%-40%) than other conventional chemotherapies, like CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone), CAP (cyclophosphamide, doxorubicin, prednisone), or chlorambucil, but did not improve overall survival when used as single agent.67-70 Similarly, cladribine monotherapy was shown to produce a higher CR rate than chlorambucil plus prednisone (47% vs 12%) without resulting in a longer survival.71
Bendamustine, 4-[5-[Bis(2-Chloroethyl)amino]-1-methylbenzimidazol-2-yl]butanoic acid was first described in 1963 by Ozegowski and Krebs.72 It was used in East Germany for treating a variety of cancers and became available in West Germany after 1990. Later, bendamustine was compared to chlorambucil in a randomized trial and produced improved responses but greater toxicity and no OS benefit.73 The overall response (OR) and median PFS rates were 67% and 22 months respectively for bendamustine vs 30% and 8 months for chlorambucil (both P < .0001). Another trial compared bendamustine to fludarabine in 96 patients with relapsed CLL requiring treatment after one previous systemic regimen.74 Overall response rates were 76% on bendamustine and 62% on fludarabine, with clinical complete response rates of 27% and 9%, respectively. Median PFS was 20.1 and 14.8 months, median overall survival 43.8 and 41.0 months. Collectively, these results suggest that bendamustine is a potent single agent for the treatment of CLL.
5.2 Monoclonal antibodies monoclonal antibodies
5.2.1 Anti-CD20 antibodies
CD20 is an activated, glycosylated phosphoprotein expressed on the surface of mature B-cells. The protein has no known natural ligand75 and acts as a calcium channel in the cell membrane. As CD20 is expressed on most B-cell malignancies, the introduction of the anti-CD20 antibody rituximab in 1998 improved the treatment of most CD20-positive non-Hodgkin lymphomas including CLL.76 Some newer CD20-antibodies challenge rituximab.77-79
Rituximab in CLL is less active as a single agent than in follicular lymphoma, unless very high doses are used.80, 81 In contrast, combinations of rituximab with chemotherapy have proven to be very efficacious therapies for CLL (see below).
Ofatumumab is a fully humanized antibody targeting a unique epitope on the CD20 molecule expressed on human B-cells. This results in increased binding affinity to CD20, prolonged dissociation rate, and increased cell killing due to greater CDC activity, and similar ADCC activity compared to rituximab, especially in cells expressing low levels of CD20.82 In a study on 201 patients that were either fludarabine-refractory and alemtuzumab-refractory (FA-refractory), or only fludarabine-refractory and suffered from bulky disease (>5 cm), ofatumumab yielded an overall response rate of 51% in the FA-refractory group, and 44% in the bulky disease group.83 On February 28, 2019, the European Commission withdrew the marketing authorisation for ofatumumab in the European Union (EU), at the request of the marketing authorisation holder, Novartis Europharm Limited, for commercial reasons.
Obinutuzumab (GA101). The humanized and glycoengineered monoclonal antibody obinutuzumab showed impressive results in vitro with higher rates of apoptosis in B-cells in comparison to rituximab.84 The humanization of the parental B-Ly1 mouse antibody and subsequent glycoengineering lead to higher affinity binding to a CD20 type II epitope, increased antibody-dependent cellular cytotoxicity (ADCC), low complement-dependent cytotoxicity (CDC) activity, and increased direct cell death induction.85 The GAUGUIN trial a phase I/II trial testing obinutuzumab monotherapy in patients with relapsed/refractory CLL patients showed that obinutuzumab was an active drug in CLL.86 Overall response rate was 62% (phase 1) and 30% (phase 2), respectively. Phase 2 median PFS was 10.7 months.
5.2.2 Other monoclonal antibodies
Alemtuzumab is a recombinant, fully humanized, monoclonal antibody against the CD52 antigen. Monotherapy with alemtuzumab has produced response rates of 33% to 53%, with a median duration of response ranging from 8.7 to 15.4 months. This was in patients with advanced CLL who were previously treated with alkylating agents, and had failed or relapsed after second-line fludarabine therapy.87-89 In addition, alemtuzumab has proven effective in patients with high-risk genetic markers such as deletions of chromosome 11 or 17 (del(11q) and del(17p)) and TP53 mutations.90, 91 Therefore, alemtuzumab is a reasonable therapeutic option for patients with these poor prognostic features. In a prospective randomized study alemtuzumab was tested against chlorambucil.92 Alemtuzumab led to a greater OR and CR (P < .0001), superior PFS with a 42% reduction in risk of progression or death (P < .0001) and significantly longer median time to progression (TTP) (P = .0001). Therefore, the drug was approved for CLL front-line therapy. Unfortunately, a strategic decision of Sanofi led to the withdrawal of the license of alemtuzumab for CLL in 2012; the drug continues to be available through an international compassionate use program.
5.3 Agents targeting the signaling in CLL cells and in their environment
B-cell receptor signaling seems to play an important role for the survival of CLL cells.93, 94 Different aspects of the B-cell-receptor have been recognized as a prognostic marker in chronic lymphocytic leukemia, such as immunoglobulin heavy chain variable gene (IGHV) mutational status or stereotypy. Continuous or repetitive BCR signaling supports CLL cell survival (reviewed in94). This might explain why inhibition of BCR signaling is a new and potent strategy to treat CLL.95 The B-cell receptor signaling in CLL cells is supported by different tyrosine kinases, such as Bruton tyrosine kinase (BTK), spleen tyrosine kinase (Syk), ZAP70, Src family kinases (in particular Lyn) as well as PI3K.95 Targeting of these B cell receptor associated kinases (BAKs), in particular of BTK or PI3K delta, by specific inhibitors has revolutionized the therapy of B lymphoid malignancies. In addition, results obtained by targeted deletion of BAKs such as Lyn and Btk in murine CLL models suggest that BAKs may also shape the dialogue between malignant B cells and the tumor microenvironment (TME).26 Since BAKs are expressed in multiple cell types, BAK inhibitors may disrupt the lymphoma supportive microenvironment.96 This concept provides a mechanistic understanding of the typical clinical response to BAK inhibitor treatment, which is characterized by a long-lasting increase of peripheral blood lymphoid cells, due to a redistribution from lymphoid homing compartments.
5.3.1 Idelalisib
Class I phosphatidylinositol 3-kinases (PI3Ks) regulate cellular functions relevant to oncogenesis.97 Expression of the PI3K p110 δ isoform (PI3K- δ) is restricted to cells of hematopoietic origin where it plays a key role in B cell proliferation and survival. In CLL the PI3K pathway is constitutively activated and dependent on PI3K δ.98 Idelalisib is an oral PI3Kδ-isoform-selective inhibitor, which promotes apoptosis in primary CLL cells. This is in a time- and dose-dependent manner without inducing apoptosis in normal T cells or natural killer cells, and without diminishing antibody-dependent cellular cytotoxicity. Idelalisib inhibits CLL cell chemotaxis toward CXCL12 and CXCL13 and migration beneath stromal cells (pseudoemperipolesis). Idelalisib also down-regulates secretion of chemokines in stromal cocultures and after BCR triggering.98 Idelalisib reduces survival signals derived from the BCR or from nurse-like cells, and inhibits BCR- and chemokine-receptor-induced AKT and MAP kinase (ERK) activation.98
In a phase 1 trial, idelalisib was evaluated in 54 patients with relapsed/refractory chronic lymphocytic leukemia (CLL) with adverse characteristics including bulky lymphadenopathy (80%), extensive prior therapy (median 5 [range 2-14] prior regimens), treatment-refractory disease (70%), unmutated IGHV (91%), and del(17p) and/or TP53 mutations (24%).99 Patients were treated at six dose levels of oral idelalisib (range 50-350 mg, once or twice daily) and remained on continuous therapy while deriving clinical benefit. The most commonly observed grade ≥3 adverse events were pneumonia (20%), neutropenic fever (11%), and diarrhea (6%). Idelalisib treatment resulted in nodal responses in 81% of patients. The overall response rate was 72%. The median PFS was 15.8 months.
5.3.2 Ibrutinib
Bruton tyrosine kinase (BTK) leads to downstream activation of cell survival pathways such as NF-κB and MAP kinases via Src family kinases.100 These pathways play a relevant role in the signal transduction of the B-cell receptor (BCR). Ibrutinib is an orally active, small molecule BTK inhibitor that induces apoptosis in B-cell lymphomas and CLL-cells.100 Fifty-six patients with relapsed or refractory B-cell lymphoma and CLL received escalating oral doses of ibrutinib, on two schedules: one, 28 days on, 7 days off; and two, once-daily continuous dosing. The ORR in 50 evaluable patients was 60%, including 16% CR. Median PFS in all patients was 13.6 months.101 The most relevant treatment-related side effects were viral infections.
Thereafter, ibrutinib was investigated in 85 patients with relapsed or refractory CLL or SLL, the majority of whom with high-risk disease.102 Fifty-one patients received a daily dose of 420 mg ibrutinib, and 34 patients received a dose of 840 mg. Side effects were predominantly grade 1 or 2 and included transient diarrhea, fatigue, and upper respiratory tract infection. Patients could receive extended treatment with minimal hematologic toxic effects. The ORR was the same in both dose groups, 420 mg and 840 mg (71%). An additional 20% and 15% of patients in the respective groups had a PR with lymphocytosis. The response was independent of clinical and genomic risk factors, including advanced-stage disease, the number of previous therapies, and the presence of a del(17p). At 26 months, the estimated PFS rate was 75% and the rate of OS was 83%. These results showed that ibrutinib yielded durable remissions in CLL/SLL patients with relapsed, refractory or high-risk disease.
Ibrutinib was compared to ofatumumab in phase 3 study.103 A group of 391 patients with relapsed or refractory CLL or SLL were included. At a median follow-up of 9.4 months, ibrutinib significantly improved progression-free survival; the median duration was not reached in the ibrutinib group (with a rate of progression-free survival of 88% at 6 months), as compared with a median of 8.1 months in the ofatumumab group (P < .001). Ibrutinib also significantly improved overall survival (P = .005). At 12 months, the overall survival rate was 90% in the ibrutinib group and 81% in the ofatumumab group.
The occurrence of resistance to ibrutinib is increasingly understood. Six initial patients with acquired resistance to ibrutinib therapy were examined by whole-exome sequencing.104 A cysteine-to-serine mutation in BTK at the binding site of ibrutinib was found in five patients and three distinct mutations in PLCgamma2 (PLCG2) were identified in two patients. Functional analysis showed that the C481S mutation of BTK results in a protein that is only reversibly inhibited by ibrutinib. The R665W and L845F mutations in PLCG2 are both potentially gain-of-function mutations leading to autonomous BCR activity.
A large single-center analysis on 308 ibrutinib-treated patients determined the features associated with discontinuation of ibrutinib therapy and subsequent outcomes.105 For patients who discontinued therapy because of disease progression, targeted deep sequencing was performed in samples at baseline and time of relapse. At a median follow-up of 20 months, 232 patients remained on therapy, 31 had discontinued because of disease progression, and 45 had discontinued for other reasons. Disease progression includes Richter's transformation (RT) or progressive CLL. Richter's transformation appeared to occur early and CLL progressions later (cumulative incidence at 12 months, 4.5% and 0.3%, respectively. Median survival following RT was 3.5 months only and 17.6 months following CLL progression. Sequencing on peripheral blood from eight patients with RT revealed two with mutations in BTK, and a lymph node sample showed no mutations in BTK or PLCG2. Deep sequencing on 11 patients with CLL progression revealed BTK or PLCG2 mutations in all. These mutations were not identified before treatment in any patient.
A later analysis of the same institution with a median follow-up time of 3.4 years showed a cumulative incidence of progression at 4 years of 19%.106 Baseline karyotypic complexity, presence of del(17) (p13.1), and age less than 65 years were risk factors for progression. Among patients who experienced relapse, acquired mutations of BTK or PLCG2 were found in 85%. These mutations were detected at an estimated median of 9.3 months before relapse. Of a group of 112 patients examined prospectively, eight patients have experienced relapse, and all of these patients had acquired resistance mutations before relapse. A resistance mutation was detected in an additional eight patients who did not meet criteria for clinical relapse. Together, these findings underscore the importance of the BCR pathway in the mechanism of action of ibrutinib in CLL. Moreover, these mutations may be detected prior to clinical relapse and serve as an anchor point for additional, targeted interventions.
5.3.3 Recent phase III trials comparing ibrutinib monotherapy with combination therapies
Several phase III studies have compared targeted agents (alone or in combination) to conventional chemoimmunotherapy (Table 2). The RESONATE-2 trial established ibrutinib monotherapy as a first line option in CLL patients by demonstrating a significant improvement of survival.107 The results were impressive, especially for CLL patients with high-risk genetics. However, the control arm (chlorambucil monotherapy) was no longer considered appropriately potent. Therefore, newer trials compared ibrutinib to more potent therapies.
| Treatment | N | Agea | ORR | CR % | PR % | uMRD % | PFSb | 2y-PFS | 2y-OS | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Randomized studies in first line treatment | ||||||||||
| Ibrutinib | 136 | 73 | 86% | 4% | 82 | NA | NR | 89% | 98% | Burger et al. 2015107 |
| Chlorambucil (CLB) | 133 | 72 | 35% | 2% | 22 | NA | 18.9 | 34% | 85% | |
| Ibrutinib + rituximab | 354 | 58 | NA | NA | NA | NA | NA | 3 years: 89% | NA | Shanafelt et al. 2018108 |
| FCR | 175 | 57 | NA | NA | NA | NA | NA | 3 years: 73% | NA | |
| Ibrutinib | 182 | 71 | 93% | 7% | NA | 1% | NR | 87% | 90% | Woyach et al. 2018109 |
| Ibrutinib + rituximab | 182 | 71 | 94% | 12% | NA | 4% | NR | 88% | 94% | |
| BR | 183 | 70 | 81% | 26% | NA | 8% | 41.0 | 74% | 95% | |
| Ibrutinib + obinutuzumab | 113 | 70 | 88% | 19% | 69% | 35% | NR | 30 m: 79% | 30 m-OS: 86% | Moreno et al. 2019110 |
| CLB + obinutuzumab | 116 | 72 | 73% | 8% | 66% | 25% | 19.0 | 30 m: 31% | 30 m-OS: 85% | |
| Venetoclax + obinutuzumab | 216 | 72 | 85% | 50% | 35% | 76% | NR | 88% | 92% | Fischer et al 2019111 |
| CLB + obinutuzumab | 216 | 71 | 71% | 23% | 48% | 35% | NR | 64% | 93% | |
| Randomized studies in treatment of relapsed/refractory CLL | ||||||||||
| BR + ibrutinib | 289 | 64 | 83% | 10% | 72% | 26% | NR | 18 m: 79% | 3y-OS: 82% | Chanan-Khan et al.112, 113 |
| BR | 289 | 63 | 68% | 3% | 65% | 6% | 13.3 | 18 m: 24% | 3y-OS: 73% | |
| Venetoclax + rituximab | 194 | 65 | 92% | 8% | 84% | 62% | NR | 85% | 92% | Seymour et al. 2018114 |
| BR | 195 | 65 | 72% | 4% | 69% | 13% | 17.0 | 63% | 87% | |
| Idelalisib + rituximab | 110 | 71 | 81% | 0 | 81% | NA | NR | 6 m: 93% | 1y-OS: 92% | Furman et al. 2014115 |
| Rituximab | 110 | 71 | 13% | 0 | 13% | NA | 5.5 | 6 m: 46% | 1y-OS: 80% | |
| BR + idelalisib | 207 | 62 | 70% | 1% | 69% | NA | 20.8 | NA | NA | Zelenetz et al. 2017116 |
| BR | 209 | 64 | 45% | 0 | 44% | NA | 11.1 | NA | NA | |
- Abbreviations: BR, bendamustine rituximab; CR %, complete response rate; FCR, fludarabine cyclophosphamide rituximab; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR %, partial response rate; uMRD %, rate of patients with undetectable MRD (<10−4) in PB.
- a median, years.
- b median, months.
The ECOG-ACRIN intergroup trial E1912 compared an indefinite ibrutinib-based treatment with the established gold standard for young and fit CLL patients, fludarabine, cyclophosphamide, and rituximab (FCR).108 The authors have claimed to show a survival benefit in patients treated with ibrutinib plus rituximab (IR) when compared to FCR. Significantly improved PFS and OS were shown in all analyzed subgroups except in IGHV mutated patients. However, the relatively short follow-up time and the very small number of events justify some caution. Moreover, a surprisingly high number of early deaths from CLL was observed in the FCR arm, raising the question of appropriate second line therapy. Woyach et al. compared ibrutinib alone or in combination with rituximab to a first-line therapy with bendamustine and rituximab (BR) for CLL patients ≥65 years of age.109 The study showed a superior PFS for ibrutinib and IR compared to BR. The addition of rituximab to ibrutinib did not result in prolonged PFS. There was no PFS advantage observed in patients with mutated IGHV. No overall survival benefit was seen for any of the arms.
The Illuminate study tested Clb-G against a combination of ibrutinib and obinutuzumab in elderly and comorbid patients.110 This combination had previously shown promising results with MRD-negative responses.117 The Illuminate study produced a significant PFS benefit for the combination of ibrutinib and obinutuzumab vs Clb-G. As the study did not contain an ibrutinib monotherapy arm, the benefit of adding obinutuzumab to ibrutinib remains unclear.
5.3.4 Acalabrutinib
Acalabrutinib (ACP-196), a potentially more selective, irreversible BTK inhibitor showed promising results with high response rates in 61 patients with relapsed CLL.118 However, the study was published at a median follow up of only 14.3 months. Therefore, more mature follow up data are needed for establishing the real clinical value of this compound.
5.3.5 Lenalidomide
Lenalidomide is a thalidomide analogue with therapeutic activity in myelodysplastic syndrome and multiple myeloma. It showed encouraging results in the treatment of high-risk CLL patients including carriers of a del(17p).119 In 58% of CLL patients lenalidomide causes a so-called tumor flare reaction, which leads to a sensation of heat and burning in the lymph nodes.120, 121 This phenomenon is much less frequently observed in other neoplasias. In CLL, the overall response rate of lenalidomide monotherapy varied between 32% and 54%.121-123 The long-term outcomes of 60 CLL patients treated with lenalidomide were reported as a single center experience.124 At a median follow-up of 4 years, time-to-treatment failure was reached, with an overall survival of 82%. Thirty-five (58%) patients had a response lasting >36 months (long-term responders [LTRs]). Best LTR responses consisted of 25 (71%) CR and 10 (29%) PR. In addition to clinical responses, an increase in IgA, IgG, and IgM levels of >50% from baseline was reported in 61%, 45%, and 42% of LTRs. Normalization in the percentage of CD4+ and CD8+ cells and T-cell numbers was observed in 48%, 71% and 99% of LTRs. Compared with other patients in the study, LTRs had lower baseline plasma levels of beta2-microglobulin, were more likely to have trisomy 12, and less likely to have a del(17p).
A promising approach seemed the use of lenalidomide as maintenance therapy in high-risk CLL. In one trial, CLL patients with at least a partial response after chemoimmunotherapy were eligible, if they had presence of minimal residual disease (at intermediate or high levels combined with an unmutated IGHV gene status or TP53 alterations).125 While this approach is able to prolong the progression-free survival substantially, it carries the risk of transformation to acute lymphoblastic leukemia and therefore cannot be recommended.
5.4 BCL-2 inhibitors
Proteins in the B cell lymphoma 2 (Bcl2) family are key regulators of the apoptotic process.126 The Bcl2 family comprises proapoptotic and prosurvival proteins. Shifting the balance toward the latter is an established mechanism whereby cancer cells evade apoptosis. B cell lymphoma 2, the founding member of this protein family, is encoded by the BCL2 gene which was initially described in follicular lymphoma as a protein in translocations involving chromosomes 14 and 18.127
5.4.1 Venetoclax
Venetoclax is a BH3-mimetic drug designed to block the function of the Bcl2 protein.128 Venetoclax inhibits the growth of BCL2 dependent tumors in vivo but spares human platelets. A single oral dose of venetoclax in three patients with refractory chronic lymphocytic leukemia resulted in tumor lysis within 24 hours.128 Therefore, a dose escalation scheme was installed to prevent these incidents,129 with a weekly dose ramp-up schedule (20, 50, 100, 200, 400 mg) over 4 to 5 weeks. Thereafter, patients should take daily 400 mg continuously dosing until disease progression or side effects occur.130 The results of a pivotal phase I/II trial were recently published.131 Overall, 56 patients received active treatment in one of eight dose groups that ranged from 150 to 1200 mg per day. In an expansion cohort, 60 additional patients were treated with a weekly stepwise ramp-up in doses as high as 400 mg per day. The majority of the patients had received multiple previous treatments, and 89% had poor prognostic clinical or genetic features. Venetoclax was active at all dose levels. Clinical tumor lysis syndrome occurred in 3 of 56 patients in the dose-escalation cohort, with one death. After adjustments to the dose-escalation schedule, clinical tumor lysis syndrome did not occur in any of the 60 patients in the expansion cohort. Other toxic effects included mild diarrhea (in 52% of the patients), upper respiratory tract infection (in 48%), nausea (in 47%), and grade 3 or 4 neutropenia (in 41%). A maximum tolerated dose was not identified. Among the 116 patients who received venetoclax, 92 (79%) had a response. Response rates ranged from 71% to 79% among patients with an adverse prognosis, including those with resistance to fludarabine, or del(17p) or unmutated IGHV. Complete remission occurred in 20%, including 5% MRD negative remissions. The 15-month PFS estimate for the 400-mg dose groups was 69%.
Another trial was conducted in 107 CLL patients with relapsed or refractory del(17p) CLL.130 At a median follow-up of 12.1 months, an overall response by independent review was achieved in 85 patients (79.4%). The most common grade 3-4 adverse events were neutropenia (43 [40%]), infection (21 [20%]), anemia (19 [18%]), and thrombocytopenia (16 [15%]). Serious adverse events occurred in 59 (55%) patients, with the most common (>/=5% of patients) being pyrexia and autoimmune hemolytic anemia (seven [7%] each), pneumonia (six [6%]), and febrile neutropenia (five [5%]). Eleven patients died in the study within 30 days of the last dose of venetoclax; seven due to disease progression and four from an adverse event (none assessed as treatment related). Taken together the results show that venetoclax is active and well tolerated in patients with relapsed or refractory del(17p) CLL, providing a new therapeutic option for this very poor prognosis population.
5.5 Pembrolizumab and checkpoint inhibition
Preclinical evidence suggested that the programmed death 1 (PD-1) pathway is critical for inhibiting the immune surveillance of CLL. Therefore, a phase 2 trial was performed with pembrolizumab, a humanized PD-1-blocking antibody, at a dose of 200 mg every 3 weeks in relapsed and transformed CLL.132 Twenty-five patients (16 relapsed CLL and 9 Richter transformations (RT)) were enrolled, 60% received prior ibrutinib. Objective responses were observed in four out of nine RT patients (44%) and in 0 out of 16 CLL patients (0%). Treatment-related grade 3 or above adverse events were reported in 15 (60%) patients and were manageable. Analyses of pretreatment tumor specimens from available patients revealed increased expression of PD-L1 and a trend of increased expression in PD-1 in the tumor microenvironment in patients who had confirmed responses. The results of this study suggest a benefit of PD-1 blockade in CLL patients with RT.
5.6 CART cells
An initial report using a lentiviral vector expressing a chimeric antigen receptor (CAR) with specificity for the B-cell antigen CD19, coupled with CD137 (a costimulatory receptor in T cells [4-1BB]) and CD3-zeta (a signal-transduction component of the T-cell antigen receptor) signaling domains showed a very impressive efficacy.133 A low dose (approximately 1.5x105 cells per kilogram of body weight) of autologous CAR-modified T cells was reinfused into a patient with refractory CLL. It expanded to a level that was more than 1000 times as high as the initial engraftment level in vivo, with delayed development of a tumor lysis syndrome and subsequent CR.
An anti-CD19 CAR-T cell therapy was applied to 24 CLL patients who had previously received ibrutinib.134 Patients received lymphodepleting chemotherapy and anti-CD19 CAR-T cells at one of three dose levels (2 × 105, 2 × 106, or 2 × 107 CAR-T cells/kg). Four weeks after CAR-T cell infusion, the overall response rate (complete response [CR] and/or partial response [PR]) was 71% (17 of 24). In 19 of these patients who were restaged, the overall response rate 4 weeks after infusion was 74% (CR, 4/19, 21%; PR, 10/19, 53%); 15/17 patients (88%) with marrow disease before CAR-T cells had no disease by flow cytometry after CAR-T cells, and seven (58%) had no malignant IGH sequences detected in marrow. Absence of the malignant IGH clone in marrow of patients with CLL, who responded by IWCLL criteria, was associated with 100% progression-free survival and overall survival (median 6.6 months follow-up). Overall, these early observations highlight the potential of CD19 CAR-T cells in CLL, but more progress needs to be achieved before recommending this modality on a broader basis.
As a principle, some of the most relevant advances in CLL treatment have been achieved by the combined use of different treatment modalities. The subsequent sections will summarize the most relevant results obtained with different drug combinations in CLL.
5.7 Combinations of different cytostatic agents
Since purine analogs and alkylating agents have different mechanisms of action and partially non-overlapping toxicity profiles, it seemed logical to combine the two modalities for achieving synergistic effects. Preclinical studies demonstrated that exposure of CLL cells to fludarabine and cyclophosphamide resulted in synergistic cytotoxicity.135 Fludarabine has been evaluated in a variety of combination regimens. The combination of fludarabine with another purine analog, cytarabine, appeared to be less effective than fludarabine alone, while the combination of fludarabine with chlorambucil or prednisone increased the hematological toxicity but not the response rate.67, 136 The most thoroughly studied combination chemotherapy for CLL is fludarabine plus cyclophosphamide (FC), which generated very promising results in phase II trials.136, 137 A Phase II study of cladribine in combination with cyclophosphamide also demonstrated activity in advanced CLL, but the results seemed inferior to FC.138
Later, three randomized trials showed that FC combination chemotherapy improves the CR and OR rate and PFS as compared to fludarabine monotherapy.139-141 The rate of severe infections was not significantly increased by the FC combination despite a higher frequency of neutropenias. A re-analysis of the CLL4 trial of the GCLLSG, suggested that the first-line treatment of CLL patients with FC combination may improve the OS of the non-high risk CLL patients (all patients not exhibiting a del(17p) or TP53 mutation).
A Polish study group compared 2-CdA alone to 2-CdA combined with cyclophosphamide (CC), or to cyclophosphamide and mitoxantrone (CMC) in 479 cases with untreated progressive CLL.142 Surprisingly, the CC or CMC combination therapies did not produce any benefit in terms of progression free survival or response rates when compared to 2-CdA alone.
5.8 Chemoimmunotherapy using rituximab
Since preclinical studies showed evidence for a synergy between rituximab and fludarabine,143 rituximab combinations with fludarabine were investigated in phase II trials. A GCLLSG trial on 31 previously treated or untreated CLL patients showed 27 (87%) responses and 10 (32%) CR.144 The CALGB 9712 protocol combined rituximab with fludarabine in either a sequential or concurrent regimen in a randomized study. Patients (n = 104) with previously untreated CLL received six cycles of fludarabine, with or without rituximab, followed by four once-weekly doses of rituximab.145 Overall and complete response rates were higher in the concurrent group (90% and 47% vs 77% and 28%). In a retrospective analysis, all patients of the CALGB 9712 protocol treated with fludarabine and rituximab were compared with 178 patients from the previous CALGB 9011 trial, who received only fludarabine.146 The patients receiving fludarabine and rituximab had a better PFS and OS than patients receiving fludarabine alone. Two-year PFS probabilities were 67% vs 45% and 2-year OS probabilities were 93% vs 81%. Similarly, a Phase II trial was conducted at the MD Anderson Cancer Center on 300 patients. They had previously untreated CLL. Rituximab plus fludarabine/cyclophosphamide (FCR) achieved an overall response rate of 95%, with CR in 72%, nPR in 10%, PR due to cytopenia in 7%, and PR due to residual disease in 6%.147 Six-year overall and failure-free survival was 77% and 51%, respectively. Median time to progression was 80 months.
These results led the GCLLSG to conduct a randomized trial, the CLL8 protocol.15 A group of 817 patients (median age 61 years) with good physical fitness were randomly assigned to receive 6 courses of fludarabine plus cyclophosphamide (FC) (n = 409), or FC plus rituximab (FCR) (n = 408). Sixty-four percent were at Binet stage B, 32% Binet C and 5% Binet A. Note, FCR induced a higher OR rate than FC (92.8% vs 85.4%) and more CR (44.5 vs 22.9) (P < .001). At 2 years PFS was 76.6% in the FCR arm and 62.3% in the FC arm (P < .01). The FCR treatment was more frequently associated with CTC grade 3 and 4 neutropenia (FCR 34%; FC 21%), while other side effects were not increased. Treatment related mortality occurred in 2.0% in the FCR and 1.5% in the FC arm. A systematic analysis of prognostic factors including molecular cytogenetics showed that the positive effect of FCR applied for most prognostic subgroups. However, FCR did not improve the survival of patients with a del(17p). Similar results were obtained in a trial comparing FCR to FC in second line treatment of CLL.148 A group of 272 patients were treated with FC and 274 patients with FCR. Overall response rates were 58% and 70% for FC and FCR, respectively, with 13% and 24.3% CR. Time to treatment failure was 20.6 vs 30.6 months.
In recent updates of the CLL8 trial, and the MD Anderson patient cohort treated with FCR, a very good outcome was demonstrated for specific subgroups of patients. They were in particular those with a mutated IGVH, del(13q), trisomy 12 or del(11q), or those patients achieving an MRD negative remission.149, 150 These patients seemed to achieve very durable remissions and a very good overall survival rate following FCR treatment. In the MD Anderson trial, a plateau was seen on the PFS curve in patients with mutated IGHV, with no relapses beyond 10.4 years in 42 patients.149
A dose-modified FCR-Lite regimen was designed to decrease the toxicity of the FCR regimen.151 This regimen reduced the dose of the two cytostatic agents, (fludarabine to 20 mg/m2 and cyclophosphamide to 150 mg/m2, days 2-4 during cycle 1, and days 1-3 in cycles 2-5) and increased the dose of rituximab (day 1 of cycle 1 at a dose of 375 mg/m2; cycles 2-5 on day 1 at 500 mg/m2 preceding chemotherapy, and on day 14 of each cycle). Maintenance rituximab at 500 mg/m2 was given every 3 months until progression. The CR rate was 77% for 50 previously untreated CLL patients with an OR rate of 100%. At a median follow-up of 2.4 years all complete responders remain in CR, except for one patient who died of a myocardial infarction while still in remission. Five patients with PRs died within 2 years of completing FCR-Lite. Grade 3/4 neutropenia was documented in only 13% of cycles, which is lower than observed with the usual FCR regimen.
More recently, it has become popular to combine bendamustine with rituximab (BR). The BR protocol was initially tested in 81 patients with relapsed CLL.152 Patients received 70 mg/m2 of bendamustine on days one and two, and 375 mg/m2 of rituximab on day one of the first cycle, and 500 mg/m2 on day one of subsequent cycles, administered every 28 days for up to six cycles. On the basis of intent-to-treat analysis, the overall response rate was 59.0%. Complete response, partial response, and nodular partial response were achieved in 9.0%, 47.4%, and 2.6% of patients, respectively. Overall response rate was 45.5% in fludarabine-refractory patients and 60.5% in fludarabine-sensitive patients. Among genetic subgroups, 92.3% of patients with del(11q), 100% with trisomy 12, 7.1% with del(17p), and 58.7% with unmutated IGHV status responded to treatment. After a median follow-up time of 24 months, the median event-free survival was 14.7 months. Severe infections occurred in 12.8% of patients. Grade 3 or 4 neutropenia, thrombocytopenia, and anemia were documented in 23.1%, 28.2%, and 16.6% of patients, respectively.
The BR regimen was also investigated as first line therapy in 117 CLL patients.153 Bendamustine was administered at a dose of 90 mg/m2 on day one and two combined with 375 mg/m2 rituximab on day 0 of the first course and 500 mg/m2 on day one during subsequent courses, for up to six courses. In all, 117 patients, age 34 to 78 years, 46.2% of patients at Binet stage C, and 25.6% of patients age 70 years or older received BR chemoimmunotherapy for first-line treatment of CLL. Overall response rate was 88.0% with a complete response rate of 23.1% and a partial response rate of 64.9%. Ninety percent of patients with del(11q), 94.7% with trisomy 12, 37.5% with del(17p), and 89.4% with unmutated IGHV status responded to treatment. After a median observation time of 27.0 months, median event-free survival was 33.9 months, and 90.5% of patients were alive. Grade 3 or 4 severe infections occurred in 7.7% of patients. Grade 3 or 4 adverse events for neutropenia, thrombocytopenia, and anemia were documented in 19.7%, 22.2%, and 19.7% of patients, respectively.
Using this information, the CLL10 study of the GCLLSG was designed to compare BR to FCR, each given for six cycles, as frontline therapy for fit CLL patients without del(17p).154 A group of 561 patients were included in the intention-to-treat population, 282 patients in the FCR group and 279 in the BR group. After a median observation time of 37.1 months median progression-free survival was 41.7 months with BR and 55.2 months with FCR, showing that BR was inferior to FCR. The number of patients achieving an MRD negative response was also higher for FCR than for BR. On the other hand, severe neutropenia and infections were more frequently observed with FCR (235 [84%] of 279 vs 164 [59%] of 278, and 109 [39%] vs 69 [25%], respectively) during the study. The increased frequency of infectious complications with FCR was more pronounced in patients >65 years. In conclusion, the CLL10 study shows that FCR remains the standard therapy in very fit CLL patients, because it yielded higher CR rates, more MRD negativity and longer PFS when compared to BR. However, elderly fit CLL patients might benefit from BR as alternative regimen.
Alemtuzumab and mitoxantrone have been added to FCR to further improve the efficacy of this regimen.155, 156 Since both regimen yielded limited improvements of therapeutic efficacy but a relevant increase of toxicity, their use is not justified outside of clinical trials. Similarly, attempts to replace fludarabine in the FCR regimen by pentostatin (PCR) failed to show statistically significant improvements of response or infection rates.157 Several other combinations have been investigated, like cladribine with rituximab, methylprednisolone plus rituximab followed by alemtuzumab, or rituximab plus alemtuzumab. Their detailed description is beyond the scope of this paper, since none of them has proven to result in higher efficacy compared to FCR.
5.9 Chemoimmunotherapy using obinutuzumab
The CLL11 protocol of the GCLLSG investigated chemoimmunotherapies with anti-CD20 antibodies combined with a milder chemotherapeutic component, chlorambucil (CLB), in previously untreated CLL patients with comorbidities.158 The rationale of this study was based on results of phase II trials using CLB in combination with rituximab (RCLB).159, 160 Moreover, encouraging results were reported in the run-in phase of the CLL11 trial on CLL patients with increased comorbidity, when treated with a combination of CLB and obinutuzumab.161 In the CLL11 trial, 781 patients with previously untreated CLL, and a score higher than six on the Cumulative Illness Rating Scale (CIRS) (range, 0 to 56, with higher scores indicating worse health status), or an estimated creatinine clearance of 30 to 69 mL per minute were assigned to receive CLB, obinutuzumab plus CLB, or rituximab plus CLB. The patients had a median age of 73 years, creatinine clearance of 62 mL/min, and a CIRS score of 8 at baseline. Treatment with obinutuzumab-CLB or R-CLB, as compared with CLB monotherapy, significantly increased response rates and prolonged PFS (median PFS, 26.7 months with obinutuzumab-CLB vs 11.1 months with CLB alone; 16.3 months with R-CLB; P < .001). Treatment with obinutuzumab-CLB, as compared with CLB alone, prolonged OS (P = .002). Treatment with obinutuzumab-CLB, as compared with R-CLB, resulted in prolongation of PFS and higher rates of complete response (20.7% vs 7.0%) and molecular response. Infusion-related reactions and neutropenia were more common with obinutuzumab-CLB than with R-CLB, but the risk of infection was not increased. Together, these results show that combining an anti-CD20 antibody with chemotherapy improved outcomes in patients with CLL and coexisting conditions. Moreover, in this patient population, obinutuzumab was superior to rituximab when combined with CLB.
5.10 Chemoimmunotherapy using ofatumumab
Given the superior preclinical activity of ofatumumab (O) compared to rituximab, it was assumed that the addition of this antibody to CLB would provide superior clinical outcomes in CLL. A randomized, open-label, phase 3 trial was conducted in 447 treatment-naïve CLL patients (median age 69 years; range 35-92) who had active disease needing treatment, but in whom fludarabine-based treatment was not possible.162 Median progression-free survival was 22.4 months in the group assigned to CLB-O arm, compared with 13.1 months in the CLB group (P < .0001). Grade 3 or greater adverse events were more common in the CLB-O group (109 [50%] patients; vs 98 [43%] given CLB alone), with neutropenia being the most common event (56 [26%] vs 32 [14%]). Grade 3 or greater infections had similar frequency in both groups. The results show that the addition of ofatumumab to chlorambucil induces a relevant extension of the progression-free survival in elderly CLL patients.
5.11 Chemoimmunotherapy using alemtuzumab
The synergistic activity of fludarabine and alemtuzumab was initially suggested by the induction of responses, including one CR, in five of six patients who were refractory to each agent alone,163 and a subsequent phase II trial showed encouraging efficacy and safety.164 The combination of alemtuzumab with rituximab has also been studied in patients with lymphoid malignancies, including those with refractory/relapsed CLL, producing an ORR of 52% (8% CR; 4% nodular PR, nPR; 40% PR).165
Two phase III trials tested alemtuzumab in combination with FC (FCA) with or fludarabine (FA). FCA showed a much higher treatment-related mortality than FCR in first line therapy, and should not be given outside of clinical trials.166 A second randomized trial compared FA to fludarabine monotherapy in previously treated patients with relapsed or refractory CLL.167 In this trial, alemtuzumab was given intravenously (i.v.). So, FA (n = 168) resulted in better PFS than fludarabine monotherapy (n = 167; median 23.7 vs 16.5 months; P = .0003), and overall survival (median not reached vs 52.9 months; P = .021) compared with fludarabine alone. Despite these interesting results, the use of this FA regimen in relapsed CLL has been widely replaced by the novel kinase or Bcl2 inhibitors.
5.12 Combinations using lenalidomide
The combination of lenalidomide and rituximab seems to increase the response rate without increasing the toxicity, even in patients with del(17p) and/or unmutated IGHV-status. In a phase II trial, 59 patients with relapsed or refractory CLL received a combination of lenalidomide and rituximab.168 In this trial, oral daily therapy with 10 mg lenalidomide was started on day nine of cycle one. Rituximab was administered at 28-day intervals for up to 12 cycles; lenalidomide could continue indefinitely if patients benefitted clinically. The overall response rate was 66%, including 12% complete responses and 12% nodular PR. Time to treatment failure was 17.4 months. The most frequent grade 3 or 4 toxicity was neutropenia (73% of patients). Fourteen patients (24%) experienced a grade 3 to 4 infection or febrile episode. In essence, this combination is a helpful alternative for patients with refractory CLL and warrants further investigation.
The combination of lenalidomide, rituximab and fludarabine in previously untreated CLL patients yielded very relevant side effects.169 The initially high toxicity rate observed with this regimen was potentially explained by a simultaneous start of all three drugs. Flinn et al.170 tested a similar treatment regimen consisting of fludarabine, rituximab and lenalidomide; three out of four patients who received all three drugs on day one experienced severe side effects. After amending the protocol, starting with lenalidomide on day eight of the first cycle, the regimen was better tolerated. This observation was later confirmed.171 Finally, the GCLLSG has investigated the combination of bendamustine, rituximab and lenalidomide (BRL) in 17 relapsed or refractory and five previously untreated CLL patients.172 The response rate was 47.1% in relapsed/refractory and 60% in untreated patients. Grade 3/4 hematological toxicity was observed in 71.4%, and severe infections in 47.6% of patients. Due to this high toxicity and the disappointingly low response rate of BRL, the trial was closed prematurely.172
5.13 Combinations using idelalisib
The PI3K delta inhibitor, idelalisib, was investigated in a multicenter, randomized, double blind, placebo-controlled, phase 3 study, in combination with rituximab vs rituximab plus placebo.115 The trial included 220 patients with decreased renal function, previous therapy-induced myelosuppression, or major coexisting illnesses to receive rituximab and either idelalisib (at a dose of 150 mg) or placebo twice daily. Patients receiving idelalisib vs those receiving placebo had improved rates of OR (81% vs 13%; P < .001) and OS at 12 months (92% vs 80%; P = .02). These results led to the approval of idelalisib and rituximab for patients with relapsed CLL who are unfit for receiving chemotherapy.
The long-term efficacy and safety of this treatment was reported in 110 patients who received at least one dose of the drug in the primary study, 75 of whom enrolled in the extension study with idelalisib monotherapy.173 The idelalisib/rituximab-to-idelalisib group had a median PFS of 20.3 months. The ORR was 85.5% (94 of 110 patients; n = 1 complete response). The median OS was 40.6 and 34.6 months for patients randomly assigned to the idelalisib/rituximab and placebo/rituximab groups, respectively. Prolonged exposure to idelalisib increased the incidence of all-grade, grade 2, and grade 3 or greater diarrhea (46.4%, 17.3%, and 16.4%, respectively), all-grade and grade 3 or greater colitis (10.9% and 8.2%, respectively) and all-grade and grade 3 or greater pneumonitis (10.0% and 6.4%, respectively) but did not increase the incidence of elevated hepatic aminotransferases.
Idelalisib was tested also in combination with ofatumumab (Idela-O) in 261 patients (median age 68 years) with three median previous therapies.174 Median progression-free survival was 16.3 months in the Idela-O group and 8.0 months in the ofatumumab group. The most frequent grade 3 or worse adverse events in the Idela-O group were neutropenia (59 [34%] patients vs 14 [16%] in the ofatumumab group), diarrhea (34 [20%] vs one [1%]), and pneumonia (25 [14%] vs seven [8%]). Serious infections were generally more common in the Idela-O group and included pneumonia (in 13% patients, sepsis in 6% and Pneumocystis jirovecii pneumonia in 5%).
These and additional data led to a warning of the FDA regarding the following toxicities, for which patients need to be monitored during under idelalisib therapy175: (a) Fatal and/or serious hepatotoxicity (in 16% to 18% of idelalisib-treated patients). (b) Fatal and/or serious and severe diarrhea or colitis (14% to 20%). (c) Fatal and/or serious pneumonitis (4%). (d) Fatal and/or serious infections (21% to 48%). (e) Fatal and serious intestinal perforation. It should be noted that patients should be monitored in particular for opportunistic infections (CMV, Pneumocystis jirovecii). This safety profile has led to a reduced use of idelalisib in CLL, although the drug has some very useful features, in particular in controlling high risk disease.176
5.14 Combinations using ibrutinib
Despite preclinical findings suggesting that ibrutinib might antagonize the antibody dependent cell killing by rituximab,177, 178 the combination of ibrutinib with rituximab was tested in patients with high-risk CLL.179 Treatment consisted of 28-day cycles of once-daily ibrutinib 420 mg together with rituximab (375 mg/m2, i.v., every week during cycle one, then once per cycle until cycle six), followed by continuous daily single-agent ibrutinib 420 mg until disease progression or until toxicities or complications precluded further treatment. A group of 40 CLL patients with high-risk disease features were enrolled, 20 of whom had del(17p) or TP53 mutations (16 previously treated, four untreated), 13 had relapsed CLL with del(11q) and seven a PFS less than 36 months after first-line chemoimmunotherapy. Toxicity was mainly mild to moderate in severity (grade 1-2). Diarrhea occurred in ten (25%) patients (grade 1 in nine patients and grade 2 in one), bleeding events in 14 (33%) patients (eight grade 1 and five grade 2), nausea or vomiting in 15 patients (38%) (ten grade 1 and five grade 2), and fatigue in seven (18%) patients (four grade 1 and three grade 2). Five patients (13%) had grade 3 infections (two lung infections, one upper respiratory tract infection, one sepsis, and one mucositis), and no grade 4 or 5 infections occurred. One patient had grade 4 neutropenia. A long-term follow-up (median 47 months) of this trial was recently reported.180 At this time, the median duration on treatment was 41 months. Overall response rate was 95%, and nine patients (23%) were reported to show a CR. Twenty-one patients discontinued treatment, 10 due to disease progression, nine for other causes, and two due to stem cell transplantation; the remaining 19 patients continue on ibrutinib. Median progression-free survival for all patients was 45 months compared to 32 months in the subgroup of patients with del(17p) (n = 21, P = .02). Fourteen patients (35%) died, five from progressive disease, five from infections, and four from other causes. Median overall survival has not been reached. Taken together, the IR combination therapy leads to durable remissions in high-risk CLL.
The HELIOS trial was a phase 3 study conducted with 578 patients with active, relapsed or refractory CLL/SLL to receive 6 courses of BR combined with either ibrutinib (420 mg daily orally) or placebo given until disease progression or unacceptable toxicity.112 At a median follow-up of 17 months, progression-free survival was significantly improved in the ibrutinib group compared with the placebo group (not reached vs 13.3 months; P < .0001). The IRC-assessed progression-free survival at 18 months was 79% in the ibrutinib group and 24% in the placebo group. The most frequent all-grade adverse events were neutropenia and nausea. A subgroup of 222 (77%) of 287 patients in the ibrutinib group and 212 (74%) of 287 patients in the placebo group reported grade 3-4 events; the most common grade 3-4 adverse events in both groups were neutropenia and thrombocytopenia. A safety profile similar to that previously reported with ibrutinib and bendamustine plus rituximab individually was noted. The results show that the addition of ibrutinib to BR results improves outcome with no new safety signals identified from the combination.
Another trial evaluated the combination of ibrutinib with ofatumumab in three different administration sequences.181 Patients with CLL/SLL, prolymphocytic leukemia, or Richter's transformation who failed at least two prior therapies were enrolled. Patients received ibrutinib 420 mg daily and 12 doses of ofatumumab 300/2000 mg in three schedules: ibrutinib lead-in (group one; n = 27), concurrent start (group two; n = 20), or ofatumumab lead-in (group three; n = 24). A group of 71 patients were included, most with del(17p) (44%) or del(11q) (31%). The most common adverse events (any grade) were diarrhea (70%), infusion-related reaction (45%), and peripheral sensory neuropathy (44%). Overall response rates in CLL/SLL patients (n = 66) were 100%, 79%, and 71% in groups one, two, and three, respectively. Estimated 12-month PFS for all patients was 89%, 85%, and 75%, respectively. The results show a good tolerability and clinical activity of this combination, with durable responses.
5.15 Combinations using venetoclax or other Bcl2-antagonists
In a first attempt to introduce Bcl2-antagonists into CLL therapies, oblimersen was tested in combination with fludarabine and cyclophosphamide in 241 CLL patients.182, 183 Fludarabine 25 mg/m2/d plus cyclophosphamide 250 mg/m2/d were administered intravenously for 3 days with or without oblimersen 3 mg/kg/d as a seven-day continuous intravenous infusion (beginning 4 days before chemotherapy) for up to six cycles. Twenty (17%) of 120 patients achieved CR/nPR in the oblimersen group and eight (7%) of 121 patients in the chemotherapy-only group (P = .025). Achievement of CR/nPR was correlated with both an extended time to progression and survival (P < .0001). The overall survival and the PFS were improved in patients that achieved at least a partial response. This study already heralded the potential of combination therapies using Bcl-2 targeted agents.
A combination of venetoclax and rituximab was investigated in 49 CLL patients with relapsed or refractory CLL or SLL and achieved encouraging results.184 Overall, 42 (86%) of 49 patients achieved a response, including a complete response in 25 (51%) of 49 patients. The two-year estimates for progression-free survival and ongoing response were 82% and 89%, respectively. Negative marrow MRD was achieved in 20 (80%) of 25 complete responders and 28 (57%) of 49 patients overall.
In a randomized, open-label, phase 3 trial, 389 patients received venetoclax for up to 2 years (from day one of cycle one) plus rituximab for the first 6 months (VR group) or bendamustine plus rituximab for 6 months (BR).114 After a median follow-up period of 23.8 months, the rate of investigator-assessed progression-free survival was significantly higher in the VR group (32 events of progression or death in 194 patients) than in the BR group (114 events in 195 patients); the two-year rates of progression-free survival were 84.9% and 36.3%, respectively. The benefit was maintained across all clinical and biologic subgroups, including the subgroup of patients with del(17p). The two-year rate of progression-free survival among patients with del(17p) was 81.5% in the VR group vs 27.8% in the BR group. The rate of grade 3 or 4 neutropenia was higher in the VR group than in the BR group, but the rates of grade 3 or 4 febrile neutropenia and infections or infestations were lower with venetoclax than with bendamustine. These results established venetoclax plus rituximab as a new second-line treatment option in CLL.
Venetoclax and obinutuzumab were initially evaluated in 12 patients with previously untreated CLL and coexisting medical conditions as part of a run-in phase of the CLL14 phase 3 protocol and showed very encouraging results.185 In particular an overall response rate of 100% and no detectable (<10−4) MRD in peripheral blood in 11 or 12 patients. The full data of the CLL14 protocol, a phase 3 comparing a fixed-duration treatment with venetoclax and obinutuzumab in patients with previously untreated CLL and coexisting conditions were recently published.111 Patients with a score of greater than 6 on the Cumulative Illness Rating Scale or a calculated creatinine clearance of less than 70 mL per minute were randomly assigned to receive venetoclax-obinutuzumab or chlorambucil-obinutuzumab. In total, 432 patients (median age, 72 years; median Cumulative Illness Rating Scale score, 8; median creatinine clearance, 66.4 mL per minute) underwent randomization, with 216 assigned to each group. After a median follow-up of 28.1 months, 30 primary end-point events (disease progression or death) had occurred in the venetoclax-obinutuzumab group and 77 had occurred in the chlorambucil-obinutuzumab group. The Kaplan-Meier estimate of the percentage of patients with progression-free survival at 24 months was significantly higher in the venetoclax-obinutuzumab group than in the chlorambucil-obinutuzumab group: 88.2% as compared with 64.1%. This benefit was also observed in patients with TP53 deletion, mutation, or both and in patients with unmutated IGHV genes. Grade 3 or 4 neutropenia occurred in 52.8% of patients in the venetoclax-obinutuzumab group and in 48.1% of patients in the chlorambucil-obinutuzumab group, and grade 3 or 4 infections occurred in 17.5% and 15.0%, respectively. One of the most important results of this trial was the very high rate of MRD negative remissions at 76% (peripheral blood) achieved in the venetoclax-obinutuzumab group.111
Two phase II trials testing combinations of venetoclax plus ibrutinib were published recently, partially with preliminary data. The CLARITY trial combined ibrutinib with venetoclax to eradicate detectable CLL in patients with relapsed or refractory CLL.186 The primary end point was eradication of MRD after 12 months of combined therapy. In 53 patients after 12 months of ibrutinib plus venetoclax, MRD negativity was achieved in the blood of 28 (53%) and the marrow of 19 (36%). Forty-seven patients (89%) responded, and 27 (51%) achieved a complete remission. After a median follow-up of 21.1 months, one patient progressed, and all patients were alive. A single case of biochemical tumor lysis syndrome was observed. Other adverse effects were mild or manageable and most commonly were neutropenia or GI events.
Another phase 2 study of combined ibrutinib and venetoclax in a total of 80 previously untreated high-risk and older patients with CLL.187 All patients had at least one of the following features: del(17p), mutated TP53, del(11q), unmutated IGHV, or an age of 65 years or older. Patients received ibrutinib monotherapy (420 mg once daily) for three cycles, followed by the addition of venetoclax (weekly dose escalation to 400 mg once daily). Combined therapy was administered for 24 cycles. Response assessments were performed according to iwCLL 2008 criteria. The MRD assessment was performed by multicolor flow cytometry in the bone marrow (sensitivity 10e-4). The median age was 65 years (range, 26 to 83). A total of 30% of the patients were 70 years of age or older. Overall, 92% of the patients had unmutated IGHV, TP53 aberration, or del(11q). After 12 cycles of combined treatment, 88% of the patients had complete remission or complete remission with incomplete count recovery, and 61% had achieved MRD negativity. Three patients had laboratory evidence of tumor lysis syndrome. Both studies highlight the potential of combining venetoclax with ibrutinib.
6 SELECTING THE RIGHT TREATMENT: HOW TO TREAT CLL?
6.1 Parameters to be considered
Given the impressive number of choices, the selection of the optimal treatment of a given CLL a patient has become a task that requires experience, a good clinical judgment and an appropriate use of diagnostic tools.
In addition to leukemia-related parameters, the newer agents may induce a number of specific effects. Therefore, the pre-existing comorbidities (eg, cardiomyopathies, arrhythmia, renal failure), the comedication (eg, CYP inhibitors, anticoagulants), and also the individual preference (time-limited vs indefinite treatment), and finally even economic considerations need to be discussed with the patient when it comes to the decision of treatment initiation.
Despite its efficacy and widespread use, indefinite ibrutinib monotherapy of CLL patients comes with some essential drawbacks: an increased financial burden, relatively high rates of cardiac arrhythmias, as well as resistance mutations and rapid relapses after discontinuation of the drug in some cases.188-190 Therefore it appears highly important to create fixed-duration combination therapies with venetoclax, ibrutinib and/or obinutuzumab that aim to achieve MRD-negative, durable responses while being safe and tolerable (Table 2).
- The clinical stage of the disease.
- The symptoms of the patient.
- The fitness and concomitant diseases of the patient, particular with regard to the potential organ toxicity of the newer, targeted agents.
- The genetic risk of the leukemia.
- The treatment situation (first vs second line, response vs non-response to the last treatment).
Using these 5 parameters, the following recommendations can be given:
6.2 First line treatment
In a patient with advanced (Binet C, Rai III-IV) or active, symptomatic disease, treatment should be initiated. In this situation, patients should be evaluated for their physical condition (or comorbidity). For patients in good physical condition (“go go”) as defined by a normal creatinine clearance and a low score at the “cumulative illness rating scale” (CIRS),191 some patients should still be offered combination therapies such as FCR, in particular when the leukemic clone shows a mutated IGVH gene (Figure 2).
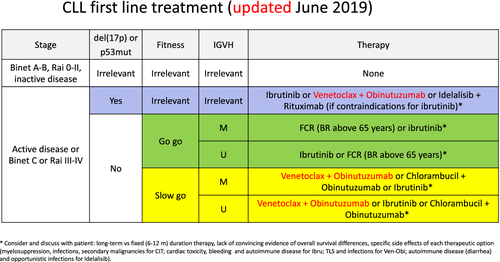
Patients with an impaired physical condition (“slow go”) may be offered either venetoclax plus obinutuzumab or ibrutinib monotherapy or chlorambucil plus obinutuzumab. Currently, there is still no clear evidence to favor one of these two options, as no survival benefit has been documented for any of these 3 options. The potential side effects (eg, atrial fibrillation, tumor lysis, autoimmune disorders) or the desire to use a fixed-duration therapy should be discussed with the patient. The aim of therapy in this situation is symptom control.
Patients with with del(17p) or TP53 mutations represent a somewhat different category. No chemoimmunotherapy should be applied but the other options (venetoclax and obinutuzumab, ibrutinib, idelalisib plus rituximab; Figure 2) usually offer a good, although not definitive disease control. In these patients, an allogeneic stem cell transplantation should be discussed at the first or second relapse.192
6.3 Second line treatment: intensification or optimal sequencing?
Figure 3 summarizes the principles of managing of patients at relapse according to the duration of remission and the physical fitness. As a general rule, the first-line therapy may be repeated, if the duration of the first remission exceeds 36 months.
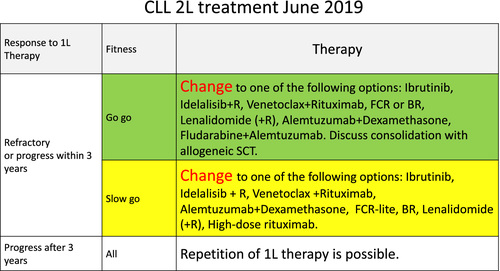
The choice of one of these options may depend on the fitness of the patient, the availability or approval status of the drugs in a given region of the world, the molecular risk profile of the leukemia, and with increasing importance on the potential side effects of the drug combinations (cardiac disease as a potential risk factor for ibrutinib; pulmonary disease and prior [CMV] infections as a potential risk factor for idelalisib etc.). According to recommendations of a European consensus group, physically fit patients with refractory CLL or with a del(17p) may be offered an allogeneic transplantation, if they relapse to one kinase inhibitor and respond to a second regimen.192 Finally, it is important to emphasize that patients with refractory disease should be treated within clinical trials whenever possible.
7 CURRENT CHALLENGES AND UNCERTAINTIES
As novel agents gain importance in the treatment of CLL, new challenges emerge when patients relapse on novel agents, and optimal sequencing strategies have not yet been established. So-called “real-world” observations suggest that ibrutinib appears superior to idelalisib when used as first kinase inhibitor.193 In the setting of ibrutinib failure, venetoclax therapy appears superior to both idelalisib and chemoimmunotherapy,193, 194 while patients refractory to venetoclax showed best outcomes when consequently treated with ibrutinib.195, 196 These data are largely derived from registries or retrospective cohort studies, lending further support for studies that optimize the sequencing strategy.
The sequenced application of single agents rarely leads to MRD-negative responses. In contrast, their combined application may induce deep and durable remissions with long-treatment-free intervals. One of these trial concepts uses sequential, targeted therapies to eradicate residual disease.32, 197 Moreover, combinations of all available drugs, as well as novel strategies to prevent clonal evolution of CLL need to be investigated198, 199 in order to achieve long-lasting remissions or even cure for CLL patients. So far, results obtained by these combination therapies appear promising, in particular when combining anti-CD20 antibodies with targeted agents.114, 117, 181, 184, 185, 200-202 While ibrutinib has been tested in combination with anti-CD20 antibodies and yielded high response rates, the choice of the antibody clearly has an impact on the efficacy. The time-limited combination treatments of ibrutinib and obinutuzumab showed an MRD-negativity rate of 48%, while ibrutinib and ofatumumab only yielded 14%. The CLL2-BAG protocol (bendamustine, venetoclax and obinutuzumab) yielded excellent overall response and MRD-negative response rates around 90% both in treatment naïve and pre-treated patients.203 Similarly, the Murano trial produced MRD negative responses in 64% of the 130 patients who completed the 24-month venetoclax plus rituximab treatment, translating into significantly longer PFS.114 Most importantly, these studies demonstrate that the majority of MRD negative remissions were sustained for more than 1 year after the end of study treatment.204, 205 In light of these results ibrutinib and venetoclax (IV) to achieve deeper remissions. Two phase II studies evaluating the use of IV have been described above.186, 187 A third trial combined the three most promising approved substances (obinutuzumab, ibrutinib and venetoclax) yielding a rate of MRD-negative responses of 70% in the treatment-naïve cohort of the study.201
When comparing these different trials for relapsed CLL patients (Table 2), it becomes evident that all combinations using targeted agents (idelalisib, venetoclax, obinutuzumab, ibrutinib) are more potent than chemoimmunotherapy. This is in regards to key variables of efficacy such overall response rate, complete remissions, MRD negative remissions, progression-free survival and overall survival. These results justify the broad use of targeted agents, alone or in combination for second-line therapy of CLL. It remains questionable, whether the addition of chemoimmunotherapy with BR to ibrutinib or idelalisib is of any substantial benefit.
Another use of kinase inhibitors may allow to enhance the function of T cells.206 It was shown that ≥5 cycles of ibrutinib therapy improved the expansion of CD19-directed CAR T cells (CTL019), in association with a decreased expression of the immunosuppressive molecule programmed cell death 1 (PD1) on T cells and of CD200 on B-CLL cells.207 Two clinical studies recently showed that this effect can be translated into higher efficacy of CAR-T cells when combined with ibrutinib, yielding high response rates and a trend toward deeper remissions compared to CAR-T cell infusions alone.208, 209
The management of CLL is continuing to undergo a very dynamic development. It is anticipated that the combination of new, non-cytostatic drugs such as obinutuzumab, ibrutinib, idelalisib or venetoclax will lead to deep, molecular and long-lasting remissions. Therefore, it is important that we hematologists and oncologists contribute to the impressive and historically unique chance to work toward the long-term control of this disease by including our patients into current clinical trials.
Moreover, in this fast-developing era of medicine bi-annually updated recommendations offer a possibility to the author and its readers to track the progress in chronic lymphocytic leukemia management.
ACKNOWLEDGEMENT
Supported by Deutsche Forschungsgemeinschaft, KFO 286.




