Comparative clinical efficacy and safety of biosimilar ABP 959 and eculizumab reference product in patients with paroxysmal nocturnal hemoglobinuria
Abstract
ABP 959 is a biosimilar to the eculizumab reference product (RP), which is approved for the treatment of patients with paroxysmal nocturnal hemoglobinuria (PNH). This multicenter, randomized, double-blind, active-controlled, two-period crossover study randomized eculizumab RP-treated patients with PNH to one of two treatment sequences (ABP 959/eculizumab RP or eculizumab RP/ABP 959) to evaluate the clinical similarity of ABP 959 when compared with eculizumab RP. This study evaluated the efficacy of ABP 959 when compared with eculizumab RP based on control of intravascular hemolysis as measured by lactate dehydrogenase (LDH) and by the time-adjusted area under the effect curve of LDH. Secondary outcomes included safety, pharmacokinetics, and immunogenicity. Forty-two patients were randomized (20 in the ABP 959/eculizumab RP group and 22 in the eculizumab RP/ABP 959 group) across 25 centers. Similarity of efficacy was established by a ratio of geometric least squares means of LDH (ABP 959/eculizumab RP) of 1.0628, with a one-sided 97.5% upper CI of 1.1576 at week 27, and a geometric means ratio of time-adjusted area under the effect curve (ABP 959 vs. eculizumab RP) of LDH of 0.981, with a 90% CI of 0.9403–1.0239 from week 13 to 27, week 39 to 53, and week 65 to 79. All secondary efficacy endpoints were comparable between treatment groups. No new safety concerns were identified. The results of this study in patients with PNH, along with previously demonstrated similarity of analytical, nonclinical, and clinical pharmacokinetics and pharmacodynamics in healthy volunteers support a demonstration of no clinically meaningful differences between ABP 959 and eculizumab RP.
Clinical Trial Registration: NCT03818607.
1 INTRODUCTION
ABP 959 is being developed as a biosimilar candidate to Soliris® (eculizumab) reference product (RP). Soliris® (eculizumab) is approved in the United States (US), European Union (EU), and other regions for four rare disease indications under the orphan designation: paroxysmal nocturnal hemoglobinuria (PNH), generalized myasthenia gravis (gMG), neuromyelitis optica spectrum disorder (NMOSD), and atypical hemolytic uremic syndrome.1, 2 Eculizumab RP, as well as ABP 959, is a recombinant, humanized, monoclonal IgG subclass 2/4 kappa (IgG2/4к) that binds to the human complement protein C5 with high affinity, thereby inhibiting its cleavage to C5a and C5b and preventing the generation of the terminal complement complex C5b-9 (also known as membrane attack complex), thus blocking complement-mediated cell lysis and activation. ABP 959 is being developed for approval in all the same indications for which the eculizumab RP has been approved.
A biosimilar is a biological product that is highly similar to a licensed RP, with no clinically meaningful differences from the originator biologic. The Food and Drug Administration's (FDA) guidance on the development and approval of biosimilars requires a totality of evidence approach to be used to generate data to support similarity between the biological product and the RP, albeit any minor inactive component differences.3, 4 The assessment of similarity begins with extensive analytical and functional characterization and is confirmed through a series of nonclinical studies, and a comparative clinical study designed to confirm that there are no clinically meaningful differences between the biosimilar and the RP. Similarly, guidelines put forth by the European Medicines Agency (EMA) require the demonstration of the similarity of the biosimilar and the RP with regard to pharmacokinetics (PK), pharmacodynamics (PD), clinical efficacy and safety, and to establish a risk management plan/pharmacovigilance plan in accordance with EU legislation.5
The development and approval of biosimilars for rare diseases has additional challenges, such as limited disease experts and treatment facilities as well as small patient populations available for enrollment in comparative clinical trials.6 Comprehensive analytical characterization has shown that ABP 959 is similar to eculizumab RP in terms of primary structure, higher order structure, posttranslational profiles, as well as purity and potency.7 Ex vivo functional assay comparisons of ABP 959 and eculizumab RP using normal and two simulated disease serums demonstrated similar complement inhibitory activity.8 PK and PD similarity as well as comparable immunogenicity and safety profiles were demonstrated in healthy adult males comparing ABP 959 with eculizumab RP (US) and eculizumab RP (EU).9 This comparative clinical study is designed to determine whether there are clinically meaningful differences between ABP 959 and eculizumab RP in efficacy, safety, tolerability, PK, and immunogenicity in eculizumab RP-treated patients with PNH.
2 MATERIALS AND METHODS
2.1 Study design
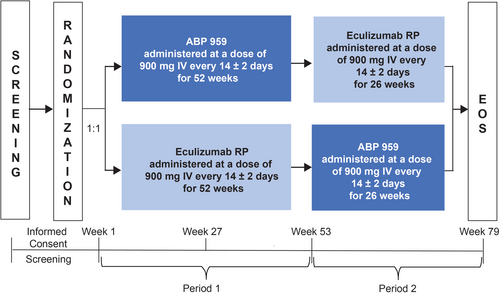
This was a randomized, double-blind, active-controlled, two-period crossover study to evaluate ABP 959 compared with eculizumab RP in adult eculizumab RP-treated patients with PNH. This study was conducted at 25 centers in the Czech Republic, Finland, France, Ireland, Italy, the Netherlands, Norway, Portugal, Slovenia, Spain, Sweden, Turkey, the United Kingdom, and the US between January 2019 and July 2022 (clinicaltrials.org: NCT03818607). Patients were randomized in a 1:1 ratio to receive each investigational product (ABP 959 and eculizumab RP) in one- of two-treatment sequences over two periods (Figure 1). Randomization occurred within 8 days prior to the first dose of the investigational product and was stratified by red blood cell (RBC) transfusion received within the last 12 months. For the ABP 959/eculizumab RP treatment group, patients received ABP 959 900 mg administered intravenously (IV) every 14 ± 2 days for 52 weeks (Period 1) followed by eculizumab RP 900 mg administered IV every 14 ± 2 days for 26 weeks (Period 2). For the eculizumab RP/ABP 959 treatment group, patients received eculizumab RP 900 mg administered IV every 14 ± 2 days for 52 weeks (Period 1) followed by ABP 959 900 mg administered IV every 14 ± 2 days for 26 weeks (Period 2). The end-of-study (EOS) visit occurred 2 weeks (±2 days) after the last dose of investigational product in Period 2. The total duration of study treatment was up to 78 weeks. At the completion of the study, patients were switched to standard-of-care non-trial eculizumab.
2.2 Study population
Eligible patients included adults ≥18 years of age with a historical diagnosis of PNH, who were stable on eculizumab RP treatment (i.e., administration of eculizumab for ≥6 months and currently receiving 900 mg of eculizumab every 14 ± 2 days). Patient eligibility required a hemoglobin ≥9.0 g/dL for at least 6 weeks before randomization, lactate dehydrogenase (LDH) <1.5 × the upper limit of normal at screening, platelet count ≥50 × 109/L, absolute neutrophil count ≥0.5 × 109/L (500 μL), and vaccination against Neisseria meningitidis. Patients were excluded from participation if they had known or suspected hereditary complement deficiency; had clinically significant cardiovascular disease, peripheral vascular disease, cerebrovascular accident, or transient ischemic attack in the previous 6 months; had evidence of acute thrombosis; were known to be positive for human immunodeficiency virus; were pregnant or breastfeeding; had a history of meningococcal infection; had presence or suspicion of active bacterial infection or recurrent bacterial infection; had a history of bone marrow transplantation; had required a RBC transfusion within 12 weeks prior to randomization; or had experienced ≥2 breakthrough events (i.e., signs and symptoms of intravascular hemolysis that required dose and/or schedule adjustments of eculizumab RP) in the previous 12 months before screening. Patients were also excluded if they were currently enrolled in or had not yet completed at least 30 days since ending another investigational study or were receiving other investigational products.
2.3 Investigational products
ABP 959, manufactured and packaged by Amgen, Inc., was formulated to contain 10 mg/mL eculizumab in acetic acid, sodium hydroxide, disodium edetate, sorbitol, polysorbate 80, and water, at pH 5.21 and supplied as a sterile, clear, colorless, preservative-free solution for IV infusion in 30-mL single-use vials. Eculizumab RP was sourced from both the US and the EU, with only US-licensed or European Economic Area-authorized reference medicinal product used in the study.1, 2 Eculizumab RP was supplied as a sterile, clear, colorless, preservative-free liquid concentrate for IV administration at a concentration of 10 mg/mL in 30-mL single-use vials. The dose and frequency of dosing were based on current eculizumab prescribing information for the maintenance treatment of patients with PNH.
2.4 Study assessments
After the screening visit, 40 additional visits were planned: 26 visits in Period 1 and 14 visits in Period 2 (Table S1). Assessments and procedures were performed as routine standard of care, prior to administration of study treatment. Assessment procedures consisted of physical examinations, vital signs, changes in concomitant medications, all adverse events (AEs), blood transfusion data, blood and urine samples for clinical laboratory testing, and baseline liver Doppler ultrasound.
2.5 Efficacy
There were two primary efficacy endpoints analyzed between treatment groups for this study to comply with study design requests from regulatory agencies. To fulfill EMA requirements, primary efficacy was measured during the parallel comparison by LDH at week 27. To fulfill the FDA requirement, primary efficacy was measured by the time-adjusted area under the effect curve (AUEC) of LDH from week 13 to 27, week 39 to 53, and week 65 to 79. Additionally, both the parallel and crossover efficacy comparisons were examined in subgroups by baseline covariates including age (≤54 vs. >54 years old), RBC transfusion received within 12 months of randomization, and gender.
Clinical laboratory secondary efficacy endpoints of total complement (CH50), total hemoglobin, serum-free hemoglobin, haptoglobin, bilirubin, degree of hemoglobinuria, type III erythrocytes, and granulocytes were summarized descriptively. Summary statistics for the number of packed RBCs (pRBC) units transfused per month were also provided. Crossover comparison of LDH at week 53 and week 79 were evaluated between treatments. Additionally, a descriptive summary of the LDH at each time point through the EOS was presented graphically.
2.6 Pharmacokinetic
The PK area under the curve (AUC) of ABP 959 and eculizumab RP and trough PK were secondary endpoints. PK assays were developed in Amgen Laboratories and transferred to third party bioanalytical labs where they were fully validated according to industry standards under the supervision of Amgen. PK AUC from week 13 to week 15 was calculated using both the total and unbound concentrations at four time points (week 13 predose, week 13 postdose, week 14, and week 15 predose).
2.7 Safety and immunogenicity
All safety analyses were performed on the safety analysis set, consisting of all treated patients with treatment assignment based on treatment received. Safety endpoints included treatment-emergent AEs, treatment-emergent serious AEs (SAEs), and treatment-emergent events of interest. Infusion reactions and serious infections were considered events of interest.
Blood samples were tested for binding antidrug antibodies (ADAs), using electrochemiluminescence bridging immunoassay. ADA assays were developed in Amgen Laboratories according to industry standards. The screening assay, utilizing an acid dissociation step, was performed on all samples. Samples with a signal-to-noise (S/N) value greater than the assay cut point were then tested to confirm specificity of the response. Drug-treated samples tested in the confirmatory assay which showed a reduction of S/N greater than the confirmatory cut point in the presence of excess soluble drug were reported as ADA positive. Samples testing positive for ADAs were also to be tested for neutralizing ADAs. The number and percentage of patients developing ADAs, neutralizing ADAs, and treatment-boosted ADAs were tabulated for each treatment.
2.8 Statistical analysis
Approximately 40 adults with PNH were to be enrolled in this study. The primary analysis of the primary efficacy endpoint of week 27 LDH was based on the full analysis set, which consisted of all randomized patients. The clinical similarity of the week 27 LDH between treatments was assessed by comparing the one-sided 97.5% upper CI limit for the GMR of LDH at week 27 with a noninferiority margin of 2.873. The point estimate of the mean difference in the log-transformed LDH and the corresponding one-sided 97.5% upper CI limit was estimated from a linear mixed effects model with treatment, stratification factor, week 1 LDH value, time (as a continuous variable), and treatment by time interaction term as fixed effects, and with the patient as a random effect. LDH values from all assessed time points from week 13 to week 27 were included in the mixed model. LDH values impacted by confounding events unrelated to the efficacy of IP, as determined by the LDH review committee, were excluded from the analysis.
Additionally, the primary analysis of the primary efficacy endpoint of the time-AUEC of LDH from week 13 to week 27, from week 39 to week 53, and from week 65 to week 79 was based on the modified full analysis set, which consisted of all randomized patients with an LDH-time profile evaluable for the time-adjusted AUEC within at least one of the 14-week assessment periods. The clinical similarity of the AUEC between treatments was assessed by comparing the two-sided 90% CI for the GMR of the time-adjusted AUEC of LDH between ABP 959 and eculizumab RP with a similarity margin of (0.77, 1.30). The point estimate of the mean difference in the log-transformed time-adjusted AUEC and the corresponding two-sided 90% CI was calculated from a linear mixed effects model with treatment, stratification factor, period, and sequence as fixed effects, and with the patient as a random effect.
Descriptive data summaries were tabulated by treatment received and summarized using the number and percentage of patients. For the crossover comparison of LDH, the full analysis set was evaluated by the mean difference in the log-transformed LDH between treatments, and the corresponding one-sided 97.5% upper CI limit was calculated from a linear mixed effects model with treatment, stratification factor, period, and sequence as fixed effects and patient as a random effect. Analyses of PK AUC were conducted using the PK parameter analysis set, which consisted of a subset of patients from the safety analysis set. Statistical assessments of total and unbound PK AUC from week 13 to week 15 were performed by initial treatment received. The point estimate and 90% CI for the GMR of ABP 959 to eculizumab RP were estimated using an analysis of variance (ANOVA). The GMR for the treatment comparison was obtained by exponentiating the difference of the means on the log scale. The 90% CI was obtained by exponentiating the CI for the difference between the means on the log scale.
3 RESULTS
A total of 47 patients were screened and 42 (20 in the ABP 959/eculizumab RP group; 22 in the eculizumab RP/ABP 959 group) were randomized and began study treatment. Demographic and baseline physical characteristics were generally comparable between the two treatment groups (Table S2). Additionally, baseline disease characteristics were generally comparable between the two treatment groups (Table S3) with a mean (SD) duration on eculizumab treatment prior to enrollment of 68.41 (39.786) months indicating a stable PNH patient population.
All 42 randomized patients were included in the full analysis set. Forty-one (97.6%) patients (20 [100.0%] in the ABP 959/eculizumab RP group and 21 [95.5%] patients in the eculizumab RP/ABP 959 group) completed Period 1 investigational product dosing. During Period 1, one patient in the eculizumab RP/ABP 959 group discontinued treatment due to an AE (asthenia and fatigue).
A total of 41 (97.6%) patients began treatment with investigational products in Period 2. Thirty-nine (92.9%) patients (19 [95.0%] in the ABP 959/eculizumab RP group and 20 [90.9%] patients in the eculizumab RP/ABP 959 group) completed Period 2 investigational product dosing and 2 (4.8%) patients (one from each group) discontinued treatment due to consent withdrawal (1 [5.0%] patient in the ABP 959/eculizumab RP group) and other (i.e., identified as patient's personal needs) (1 [4.5%] patient in the eculizumab RP/ABP 959 group). No patients discontinued investigational products in either period due to COVID-19-related reasons.
3.1 Efficacy
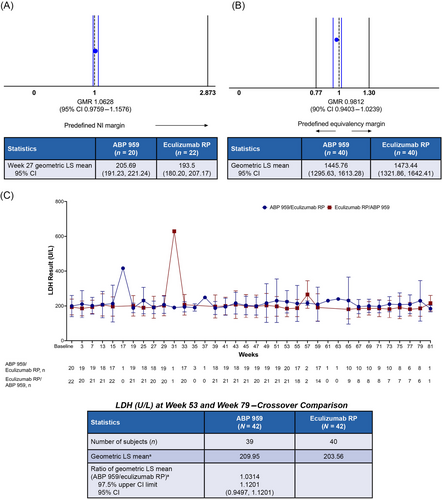
Similarity of primary and secondary efficacy was established. In the parallel comparison, the GMR of LDH at week 27 (ABP 959 vs. eculizumab RP) was 1.0628, with a one-sided 97.5% upper CI of 1.1576 contained within the noninferiority margin of 2.873 (Figure 2A). In the crossover comparison, a point estimate of the GMR of time-adjusted AUEC of LDH (ABP 959 vs. eculizumab RP) of 0.9812 with a two-sided 90% CI of (0.9403–1.0239) was contained within the prespecified margin of similarity (0.77, 1.30; Figure 2B).
To assess the robustness of the primary efficacy analyses, sensitivity analyses were conducted to examine the potential difference in outcomes when including LDH values that were originally excluded mainly due to hemolysis in the tube with no other signs of worsening PNH, one due to cholecystitis, one due to an upper respiratory infection, and three due to COVID19. The sensitivity analysis for the parallel comparison included an additional four LDH values in four patients in the ABP 959/eculizumab RP group and two LDH values in two patients in the eculizumab RP/ABP 959 group and confirmed non-inferiority of ABP 959 when compared with eculizumab RP (Table S4). The sensitivity analysis for the crossover comparison included an additional 12 LDH values for nine patients in the ABP 959/eculizumab RP group and seven LDH values for five patients in the eculizumab RP/ABP 959 group and confirmed efficacy similarity between ABP 959 and eculizumab RP (Table S5). Additional results of subgroup analyses by baseline covariates including age (≤54 vs. >54 years old), RBC transfusion received within 12 months of randomization, and gender were also consistent with the primary efficacy analysis for both the parallel comparison and crossover comparison (data not shown).
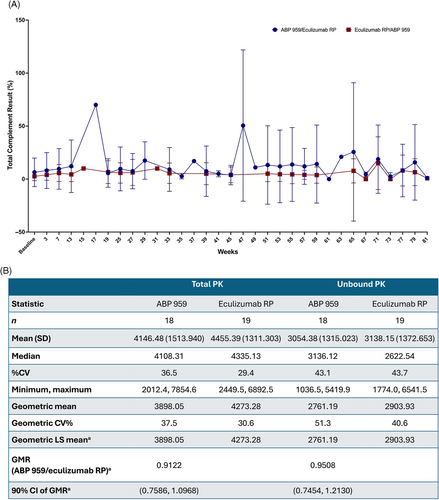
Secondary efficacy endpoints were comparable between the treatment groups. Mean values for total complement activity (CH50), total hemoglobin, serum-free hemoglobin, haptoglobin, bilirubin, degree of hemoglobinuria, type III erythrocytes, and granulocytes were stable over time and comparable between the treatment groups (Table S6). Mean CH50 values over the entire study are shown graphically in Figure 3A. Several patients in the ABP 959/eculizumab RP group had higher CH50 values prior to ABP 959 dosing, resulting in higher mean values for the ABP 959/eculizumab RP group throughout the study. Of note, the mean values remained high in these patients following the switch to eculizumab RP at week 53. Overall, CH50 values were well suppressed across both treatment groups, and the slight differences in mean values were not considered to be clinically meaningful. The mean number of the units of pRBCs transfused during the study was also comparable between the treatment groups (Table S7). The secondary efficacy endpoint for the crossover comparison of hemolysis, as measured by LDH at week 53 and week 79, resulted in the ratio of the geometric least squares means of LDH (ABP 959 vs. eculizumab RP) of 1.0314, with a one-sided 97.5% upper CI of 1.1201 and a 95% CI of (0.9497, 1.1201). The overall mean values of the LDH time profile from baseline through EOS were stable over time and comparable between the treatment groups (Figure 2C).
3.2 PK
The GMR (90% CI) for the total and unbound PK AUC from week 13 to week 15 was 0.9122 (0.7586, 1.0968) and 0.9508 (0.7454, 1.2130), respectively (Figure 3B). Geometric mean values for trough total and trough unbound concentrations of ABP 959 and eculizumab RP were similar between the treatment groups at all time points tested over the entire study.
3.3 Safety and immunogenicity
All 42 randomized patients were treated with investigational products and included in the safety analysis set. For the safety analyses, patients were included in each group if they received at least one dose of the respective investigational product in Period 1 or Period 2; AEs were assigned to the treatment that the patient was receiving at the time of the event (Table 1). Through EOS, 33 (80.5%) patients receiving ABP 959 and 39 (92.9%) patients receiving eculizumab RP reported at least one AE, most AEs were Common Terminology Criteria for Adverse Events grade 1 or 2. Eight (19.5%) patients receiving ABP 959 and 12 (28.6%) patients receiving eculizumab RP experienced Common Terminology Criteria for Adverse Event grade ≥3 AEs. For patients receiving ABP 959 (21 [51.2%]) and patients receiving eculizumab RP (21 [50.0%]), the highest incidence rate of AEs was in infections and infestations. There were no differences in the frequency of AEs by system organ class by treatment. Of note, the number of patients who experienced an incidence of hemolysis was 2 (4.9%) when receiving ABP 959 and 4 (9.5%) when receiving eculizumab RP. All frequently reported AEs through the EOS were expected per eculizumab RP labeling.1 The incidence of SAEs was seven (17.1%) patients receiving ABP 959 experienced 14 SAEs, and two (4.8%) patients receiving eculizumab RP experienced five SAEs. SAEs for patients receiving ABP 959 included cardiac failure, cholecystitis, gastroenteritis, vertigo, anemia, COVID-19, knee meniscus injury, and depression; while SAEs for the patients receiving eculizumab RP included anemia, and cardiac failure. Although more SAEs were reported by patients receiving ABP 959 compared with patients receiving eculizumab RP, these events were determined to be either confounded by other factors or are events known to occur in the PNH population. A review of individual events by the principal investigators did not identify any new safety concerns or new patterns of SAEs; therefore, these numeric differences were not conclusive of a difference in the safety profile between ABP 959 and eculizumab RP.
| Adverse event category | ABP 959 n (%) | Eculizumab RP n (%) |
|---|---|---|
| N | 41 | 42 |
| Any adverse event | 33 (80.5) | 39 (92.9) |
| Any grade ≥3 adverse event | 8 (19.5) | 12 (28.6) |
| Any fatal adverse event | 0 (0.0) | 0 (0.0) |
| Any serious adverse event | 7 (17.1) | 2 (4.8) |
| Any adverse event leading to discontinuation of IP/study | 0 (0.0) | 1 (2.4) |
| Any event of interest | 17 (41.5) | 15 (35.7) |
| Infusion reaction adverse eventa | 15 (36.6) | 15 (35.7) |
| Serious infection adverse eventb | 3 (7.3) | 0 (0.0) |
| Antidrug antibody (ADA) results | ABP 959/eculizumab RP (N = 20) | Eculizumab RP/ABP 959 (N = 22) |
|---|---|---|
| Patients with a postbaseline result through EOS | 20 | 22 |
| Treatment-boosted antibody incidence, n (%) | ||
| Binding antibody positive at baseline with a ≥4 times increase in magnitude postbaseline | 0 (0.0) | 0 (0.0) |
| Transientc | 0 (0.0) | 0 (0.0) |
| Developing antibody incidence, n (%) | ||
| Binding antibody positive postbaseline with a negative or no result at baseline | 0 (0.0) | 2 (9.1) |
| Transientc | 0 (0.0) | 2 (9.1) |
| Neutralizing antibody positive postbaseline with a negative or no result at baseline | 0 (0.0) | 0 (0.0) |
| Transientc | 0 (0.0) | 0 (0.0) |
- Note: One patient in the eculizumab RP treatment group discontinued IP/study due to adverse events of grade 2 non-serious asthenia and grade 2 non-serious fatigue. The patient discontinued the study prior to treatment crossover, as reflected in the ABP 959 group N = 41. Only treatment-emergent adverse events (AEs) were summarized. For each category, patients were included only once, even if they experienced multiple events in that category. Baseline was defined as the last non-missing assessment taken prior to the first dose of study investigational product. Percentages are calculated using the corresponding category count as the denominator. Serious AEs for patients receiving ABP 959 included cardiac failure, cholecystitis, gastroenteritis, vertigo central nervous system origin, anemia, COVID-19, meniscus injury, and depression; while serious AEs for the patients receiving eculizumab RP included anemia, cardiac failure, and cardiac failure.
- Abbreviations: EOS, end of study; IP, investigational product.
- a Identified using the hypersensitivity Standardized Medical Dictionary for Regulatory Activities Query (broad) and infusion reaction Amgen Medical Dictionary for Regulatory Activities Query (broad) search strategies.
- b Identified using the infections and infestations system organ class (broad) search strategy.
- c Negative result at the patient's last time point tested within the study period.
Through the EOS, the number of patients reporting any event of interest (infusion reactions and serious infections) was 17 (41.5%) for ABP 959 and 15 (35.7%) for eculizumab RP. The incidences of infusion reactions were 15 (36.6%) and 15 (35.7%) patients, respectively. The incidences of serious infections were three (7.3%) and 0 (0.0%) patients, respectively. There were no clinically relevant differences in the incidence or nature of event of interest between the treatment groups.
Immunogenicity data are organized by treatment received (Table 1). At baseline, no patient in either treatment group tested positive for preexisting binding ADAs or neutralizing ADAs. Through the EOS, all patients had at least one on-study ADA result, with two (9.1%) patients in the eculizumab RP/ABP 959 treatment group testing positive for binding ADAs following the crossover in treatment to ABP 959. The results were transient for both patients and neither patient experienced a SAE related to the transient ADAs. Through EOS, no patients in either treatment group tested positive for neutralizing ADAs or treatment-boosted ADAs.
4 CONCLUSIONS
Similarity in clinical efficacy was established between ABP 959 and eculizumab RP. This randomized, double-blind, two-period crossover, 18-month, clinical trial was designed, with guidance from both the FDA and EMA, to compare the efficacy and safety of ABP 959 and eculizumab RP in patients with PNH. The first analysis was performed during the parallel comparison to satisfy EU regulatory requirements, and a second analysis was completed during the crossover comparison to satisfy US regulatory requirements. At the time of study design, PNH and atypical hemolytic uremic syndrome were the only two known approved indications for eculizumab RP. PNH is a rare acquired hematologic disorder that is estimated to affect one–two individuals per million in the general population. In patients with PNH, RBC destruction releases LDH, causing elevated serum LDH levels. Based on clinical observations, increased levels of LDH (≥1.5 × the upper limit of normal) are associated with an increased risk for thrombosis, therefore, LDH is an accepted marker of RBC destruction and a clinically meaningful endpoint.10-12 This relatively large study population of adult patients with PNH who were stable on eculizumab treatment was determined to be an appropriate and homogeneous study population in terms of expected response to eculizumab treatment to provide the essential information required.
The primary efficacy endpoint for the parallel comparison was hemolysis, measured by LDH at week 27. The timepoint of week 27 was chosen for the analysis of LDH in the parallel comparison because assessment at this time point is more than sufficient to eliminate the possibility of carryover effects of drug exposure from prior eculizumab RP treatment in patients with stable PNH and allows sufficient time for LDH response to ABP 959, thus, patients will have reached a steady state of LDH following the change of treatment. The parallel comparison was designed and then demonstrated through a one-sided analysis that ABP 959 was noninferior to eculizumab RP. The primary efficacy endpoint for the crossover comparison was hemolysis, measured by the AUEC of LDH. The crossover comparison was designed and then demonstrated through a two-sided analysis that ABP 959 was non-inferior and non-superior to eculizumab RP. These analyses established a similarity of the efficacy of ABP 959 when compared to eculizumab RP in patients with PNH. Similarity of efficacy was further confirmed with results from all secondary efficacy endpoints between the two treatment groups in which total complement (CH50), total hemoglobin, serum-free hemoglobin, haptoglobin, bilirubin, type III erythrocytes, and granulocytes were stable and comparable between ABP 959 and eculizumab RP over the study period. The secondary efficacy crossover comparison of hemolysis as measured by LDH, as well as the mean values of LDH at baseline through EOS, and the mean number of pRBC units transfused per month were stable over time, and the values were comparable between the two treatment groups.
Clinical PK, safety, and immunogenicity similarity were previously demonstrated in a double-blind, randomized PK/PD study between ABP 959 and eculizumab RP in healthy adult males.9 This study confirmed clinical PK, safety, and immunogenicity similarity in a patient population with PNH. Clinical PK between ABP 959 and eculizumab RP was supported by assessments of total and unbound PK AUC of ABP 959 and eculizumab RP from week 13 to week 15 as well as geometric mean values for trough total and unbound serum concentrations at all time points tested over the entire study. Furthermore, no new safety concerns were identified in this study. Treatments of ABP 959 and eculizumab RP were well-tolerated and confirmed a similar safety profile. The immunogenicity profile was also similar with no clinically meaningful differences between the two treatment groups and no neutralizing ADAs or treatment-boosted ADAs detected in any patient. Although some rates of certain AEs were noted to be different between the treatment groups, the differences were not clinically meaningful from a safety standpoint. All frequently reported AEs through the EOS were anticipated per the eculizumab RP labeling.1, 2
Overall, this randomized, double-blind comparative clinical trial demonstrated comparative similarity in efficacy and safety of ABP 959 and eculizumab RP in patients with PNH. The overall safety and immunogenicity were similar between ABP 959 and eculizumab RP over the entire study period and were not affected by the switch in treatment products at either the beginning of the study or at week 53. The results presented in this study add to the totality of scientific evidence of no clinical difference between ABP 959 and eculizumab RP.
FUNDING INFORMATION
This research was funded by Amgen Inc.
CONFLICT OF INTEREST STATEMENT
AK is a consultant for Samsung, Novo Nordisk, Alexion/AstraZeneca, Arrowhead, Silence Therapeutics; receives research support to their institute from Celgene/BMS and Novartis; speaker's fees from Alexion/AstraZeneca, Amgen, Agios, Celgene/BMS, Pfizer, Novartis, Ra Pharma/UCB, Roche, SOBI, Janssen; and serves on scientific advisory boards/data monitoring committees for Alexion/Astra Zeneca, Amgen, Agios, Biocryst, Celgene/BMS, Novartis, Pfizer, Regeneron, Roche, SOBI, Janssen. FL is a consultant for Sobi, Roche, ABVIE, Amgen, and Novartis and received funding from Pfizer and Alexion. AA has received honoraria as a consultant and/or speaker and has received research grants from Takeda/Shire and Bayer, as well as speaker's fee and consultant for Sobi, Sanofi, Alexion Amgen, and Grifols. SC is a consultant and serves on the advisory board/steering committee for Agios, Alexion, Amgen, Roche/Genentech and Takeda; and receives research support from Alexion, Agios, Amgen, GBT/Pfizer, Forma Therapeutics, Novartis, and Roche/Genentech. SL, AT, and VH report no conflicts of interest. JC, MJM, AC, BS, DTM, VC, and HH are employees and stockholders of Amgen.
MEDICAL WRITING ASSISTANCE
Medical writing assistance was provided by Jennifer L. Fogel, PhD (Amgen Inc.) under the guidance of Sonya G. Lehto, PhD (Amgen Inc.). Editing and design support were provided by Innovation Communications Group, New York, NY (on behalf of Amgen Inc.).
PATIENT CONSENT STATEMENT
All subjects were to provide written informed consent prior to entering the study and before initiation of any study-related procedure (including administration of investigational product). The investigator was responsible for explaining the benefits and risks of participation in the study to each subject or the subject's legally acceptable representative and for obtaining written informed consent.
Open Research
DATA AVAILABILITY STATEMENT
There is a plan to share data. This may include deidentified individual patient data for variables necessary to address the specific research question in an approved data-sharing request also related data dictionaries, study protocol, statistical analysis plan, informed consent form, and/or clinical study report. Data sharing requests relating to data in this manuscript will be considered after the publication date and (1) this product and indication (or other new use) have been granted marketing authorization in both the US and Europe or (2) clinical development discontinues and the data will not be submitted to regulatory authorities. There is no end date for eligibility to submit a data sharing request for these data. Qualified researchers may submit a request containing the research objectives, the Amgen product(s) and Amgen study/studies in scope, endpoints/outcomes of interest, statistical analysis plan, data requirements, publication plan, and qualifications of the researcher(s). In general, Amgen does not grant external requests for individual patient data for the purpose of reevaluating safety and efficacy issues already addressed in the product labeling. A committee of internal advisors review requests. If not approved, a Data Sharing Independent Review Panel may arbitrate and make the final decision. Requests that pose a potential conflict of interest or an actual or potential competitive risk may be declined at Amgen's sole discretion and without further arbitration. Upon approval, information necessary to address the research question will be provided under the terms of a data sharing agreement. This may include anonymized individual patient data and/or available supporting documents, containing fragments of analysis code were provided in analysis specifications. Further details are available at the following: http://www.amgen.com/datasharing.




