Feasibility of a pediatric long-term Home Ventilation Program in Argentina: 11 years' experience
Abstract
Background
Pediatric home ventilation (HV) has increased worldwide. A Home Ventilation Program (HVP) was started in the Pulmonary Department of the “Hospital de Pediatría Prof. Dr. J. P. Garrahan,” Argentina, in 2007. This is the largest Argentine national pediatric tertiary care referral center. Limited studies on pediatric HV from Latin American countries have been published.
Objective
This study describes and analyzes the cohort of children admitted to the HVP during an 11 years period.
Methods
Longitudinal study. Population: all patients (pts) admitted to the HVP between 2007 and 2018. We analyzed demographic and clinical variables, sleep study results, ventilation setting, and start manner collected in a prospective data base.
Results
A total of 244 pts were admitted. Median age at ventilation start was 9.41 (3.47-14.08) years, 84% of pts had health insurance. The most frequent underlying diseases were neuromuscular disease (43%) and genetic syndromes (23%). Home-hospital distance was 100-500 km in 16% of cases and greater than 500 km in 34%. Seventy percent of pts had sleep studies before ventilation initiation. Ventilation was started in our general pediatric ward in 83.6%. Noninvasive ventilation was used in 86.1%. The actual number of pts still on follow up is 133 of 244 (54.5%), 16.8% dropped out, 16.4% were transitioned to adult care, 5.32% resolved their sleep-disordered breathing, and 5.32% died.
Conclusions
The HVP admitted pts from all the country. Ventilation was started on the basis of clinical and objective sleep measures. This long-term experience underlines the feasibility of a HVP in an emergent country.
Abbreviations
-
- AH
-
- isolated nocturnal alveolar hypoventilation
-
- ARF
-
- acute respiratory failure
-
- CAI
-
- central apnea index
-
- CCHS
-
- congenital central hypoventilation syndrome
-
- CPAP
-
- continuous positive airway pressure
-
- CSA
-
- central sleep apnea
-
- H
-
- isolated hypoxemia
-
- HV
-
- home ventilation
-
- HVP
-
- Home Ventilation Program
-
- LTHV
-
- long-term home ventilation
-
- MMC
-
- myelomeningocele
-
- NICU
-
- neonatal intensive care unit
-
- NIV
-
- noninvasive ventilation
-
- NMD
-
- neuromuscular disease
-
- OAHI
-
- obstructive apnea-hypopnea index
-
- OSAS
-
- obstructive sleep apnea syndrome
-
- PICU
-
- pediatric intensive care unit
-
- PSG
-
- polysomnography
-
- pts
-
- patients
-
- RP
-
- respiratory polygraphy
-
- SDB
-
- sleep-disordered breathing
-
- TST
-
- total sleep time
1 INTRODUCTION
Home ventilation (HV) as a treatment option for children with chronic respiratory failure is increasing worldwide. Limited data from South American countries are available in the literature. Survival of children with chronic diseases and requirements for long-term home ventilation (LTHV) raised the need for a home ventilation program (HVP). In 2007, the HVP was started in the Pulmonary Department of the “Hospital de Pediatría Prof. J. P. Garrahan,” Buenos Aires, Argentina. The hospital is the largest Argentine national pediatric tertiary care referral center. It has a total of 588 beds, of which 77.5% are general ward beds, 12.7% are intensive care beds, and 9.7% are neonatal care beds. The HVP is an interdisciplinary program and includes pulmonary and sleep physicians, a general pediatrician, nutritionists, mental health specialists, respiratory therapists, sleep technicians, and nurses.
Argentina is considered an emerging country, nevertheless 27% of the total population live under the poverty line.1
It has a fragmented and segmented health system divided into three large sectors, public, social security, and the private sector as it happens to be in many other Latin American countries. Sixty percent of the population relies on health insurance from social security, 35% depends on public health and 5% has a private insurance.2
The social security sector consists of many different sick funds: the obras sociales (some of them operate nationwide and are mostly managed by trade unions, and others include all public employees in each province) and the Programa de Asistencia Médica Integral for people with disabilities and retired workers and pensioners.
This paper aims to describe and analyze the 11 years' experience of a HVP in the Argentine's National Pediatric Tertiary Care Referral Center.
1.1 Primary objective
-
To describe and analyze the cohort of children admitted to our HVP, including demographic and clinical characteristics, sleep studies, and clinical and technical aspects related to ventilation start and outcomes.
2 METHODS
Population: All patients (pts) admitted to the program during the period January 2007- January 2018 were included for analysis.
Methods: We performed an analysis of longitudinal data collected in a prospective database. A datasheet was completed and entered into the Excel database on the day of discharge from inpatient training and during all consecutive visits to the HVP. At the end of the first 11-years follow-up period, we analyzed the number of pts admitted per year to the program, the growth rate of the program, demographic data including age, sex, distance from the home area to the hospital, and health insurance coverage. The underlying diseases were grouped into major categories and subcategories. Ventilation is routinely started in the HVP when sleep-disordered breathing (SDB) is confirmed on sleep study results or on the basis of clinical status in pts with normal sleep studies or without sleep studies.
For the purpose of this analysis, in pts whom ventilation was started based only on their clinical status, the latter was classified as: history of recurrent atelectasis or respiratory infections with acute respiratory failure (ARF), weaning failure, perioperative face of spinal surgery in neuromuscular disease (NMD), failure to thrive, diurnal hypercapnia in the arterial blood gas analysis (paCO2 > 45 mm Hg), and other causes.
-
Group 1: obstructive sleep apnea syndrome (OSAS) if the obstructive apnea-hypopnea index (OAHI) was greater than 5 (moderate OSAS when OAHI was between 5 and 9.9 and severe OSAS when OAHI was ≥10).
-
Group 2: central sleep apnea (CSA) if the central apnea index (CAI) was ≥5 in pts between 6 and 12 months old and ≥3 in pts older than 12 months.
-
Group 3: OSAS + CSA as previously defined. Associated hypoxemia and/or hypercapnia in groups 1, 2, or 3 was considered when mean SaO2 was less than 95% or total sleep time (TST) with SaO2 < 90% ≥ 2% and/or CO2 was greater than 50 mm Hg during more than 25% of TST.
-
Group 4: isolated hypoxemia (H); included pts with mean SaO2 less than 95% or mean SaO2 greater than 95% but with TST with SaO2 < 90% ≥ 2% (without OSAS, CSA, or nocturnal alveolar hypoventilation).
-
Group 5: isolated nocturnal alveolar hypoventilation (AH) was defined as the presence of CO2 greater than 50 mm Hg during more than 25% of TST without confirmation of OSAS or CSA with or without hypoxemia.
We also analyzed which was the prevalent sleep-disordered breathing (SDB) in the most frequent underlying diseases.
-
electively during a programmed in-pts training week
-
subacutely, the day after an abnormal sleep study
-
acutely in the context of an ARF
Routinely, in the HVP, when ventilation is started in a programmed manner, pts are admitted for a short stay for a training period with their parents. A multidisciplinary team trains pts and families in ventilator use, alarm responses, equipment maintenance, and recognition of unstable clinical situations.
The place where it was started was classified into general pediatric ward, the pediatric intensive care unit (PICU), neonatal intensive care unit (NICU), or referred from other hospitals.
We also analyzed whether ventilation was invasive or noninvasive and which was the ventilatory mode used: continuous positive airway pressure (CPAP), bilevel, pressure-control mode, or hybrid modes (AVAPS) when admitted to the program.
Finally, the number of pts still on follow up at the end of the study period was analyzed and the reasons to stop follow up classified into drop out (defined as being absent or lost from the HVP for more than a year), transition to adult care or transferred to other centers, SDB resolved and died. Statistics: data were analyzed using STATA 12. Frequencies, mean and standard deviation or median and interquartile ranges were used.
Approval was obtained from the Institutional Research Department.
3 RESULTS
A total of 244 pts were admitted between January 2007 and January 2018. Table 1 summarizes demographic data. Median age (years) at ventilation initiation was 9.41 years (3.47-14.08). Eighty-four percent of the children admitted to the HVP had health insurance, of which two-thirds belonged to their parents' social workers' health insurance and one-third was provided due to disability.
| Demographic characteristics | n 244 |
|---|---|
| Median age at initiation years (IQR) | 9.41 (3.47-14.08) |
| Sex distribution (F/M) | 96/148 |
| Health insurance n (%) | 205 (84.02%) |
| Workers social insurance | 145 (70.73) |
| Disability | 60 (29.27) |
| Financial support for equipment | n (%) |
| Social insurance | 194 (79.51) |
| Fundación Garrahan | 33 (13.52) |
| Local regional administration | 15 (6.15) |
| Family | 2 (0.82) |
| Distance to the hospital, km | n (%) |
| <10 | 17 (6.97%) |
| <100 | 105 (43.03%) |
| 100-500 | 38 (15.57%) |
| >500 | 84 (34.43%) |
- Abbreviation: IQR: interquartile range.
Table 2 details specific underlying disease groups and their subcategories.
| Disease categories and subcategories | n (%) |
|---|---|
| NMD | 105 (43.03) |
| Spinal muscular atrophy | 41 (16.8) |
| Duchenne muscular dystrophy | 21 (8.61) |
| Myopathy | 19 (7.79) |
| Collagen VI deficiency | 8 (3.28) |
| Merosin deficiency muscular dystrophy | 7 (2.87) |
| Other NMD | 9 (3.69) |
| Genetic syndromes | 56 (23) |
| Achondroplasia | 12 (4.92) |
| Prader-Willi | 7 (2.87) |
| Crouzon + Pfeiffer | 5 (2.04) |
| Mucopolysaccharidosis | 4 (1.64) |
| Down | 2 (0.82) |
| Treacher-Collins | 2 (0.82) |
| Other | 24 (9.84) |
| Myelomeningocele | 22 (9.02) |
| Obesity | 18 (7.38) |
| CCHS | 11 (4.51) |
| CNS tumor | 7 (2.87) |
| Chronic lung disease | 4 (1.64) |
| ROHHAD | 4 (1.64) |
| Other diseases | 17 (6.97) |
- Abbreviations: CCHS, congenital central hypoventilation syndrome; CNS, central nervous system; NMD, neuromuscular disease; ROHHAD, rapid-onset obesity with hypothalamic dysregulation, hypoventilation, and autonomic dysregulation.
Median age (years) at ventilation onset according to underlying diseases was 10.99 (4.83-14.61) in NMD, 5.70 (2.59-12.68) in genetic syndromes, 0.24 (0-0.49) in congenital central hypoventilation syndrome (CCHS), 5.99 (3.86-6.69) in rapid-onset obesity, hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (ROHHAD), 11.27 (5.86-13.48) in myelomeningocele (MMC), 10.19 (6.60-14.01) in obesity hypoventilation syndrome, and 12.44 (10.44-16.04) in central nervous system (CNS) tumors.
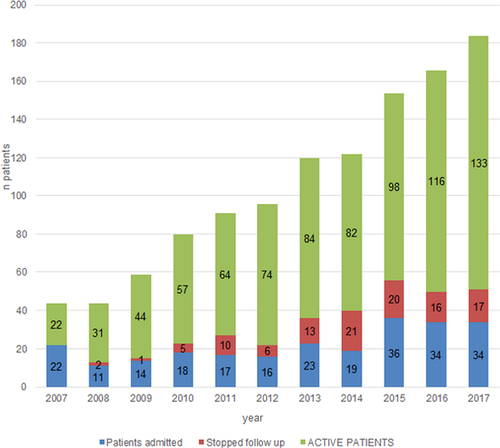
Figure 1 shows the growth rate of the program with details about the number of new pts, of those who were no longer in follow up, and the total number still in follow up per year. In 2007, the number of total pts was 20: 10 pts had started ventilation that year and 10 pts had started between 2003 and 2007. The number of new pts admitted per year and the total number of pts on follow up increased progressively except for the last 2 years, in which there is a plateau in the number of new pts admitted per year, as well as an increase in the number of pts who were no longer in the follow up.
A total of 173 of 244 (70.9%) pts had sleep studies, 157 of 173 (90.7%) PSG, 13 of 173 (7.51%) nocturnal oxicapnography, and 2 (1.15%) diurnal ABG with nocturnal oximetry. Simplified studies (oxicapnography or oximetry) were performed in pts who denied snoring or apnea.
The reasons to initiate ventilation are shown in Figure 2. In 79 of 244 (32.38%) pts, ventilation initiation was decided on the basis of their clinical status, 71 without sleep studies, and 8 with normal sleep studies. Their disease category was 60 of 79 (77.6%) NMD, 7/79 (8.86%) CCHS, 1 of 79 (1.26%), ROHHAD syndrome, and 11 (13.9%) other diseases. In 165 of 244 (67.62%) pts, abnormal findings in sleep studies confirmed SDB and, along with clinical symptoms were the reason to start ventilation. SDB distribution in the pts with PSG is shown in the graph.
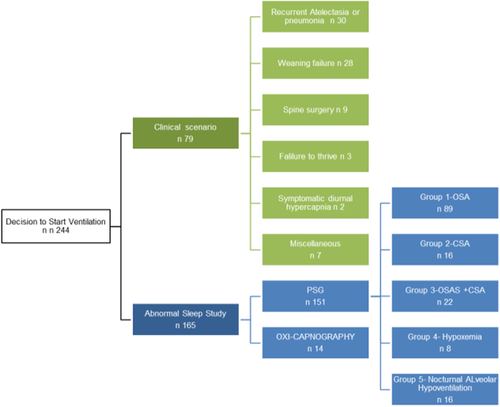
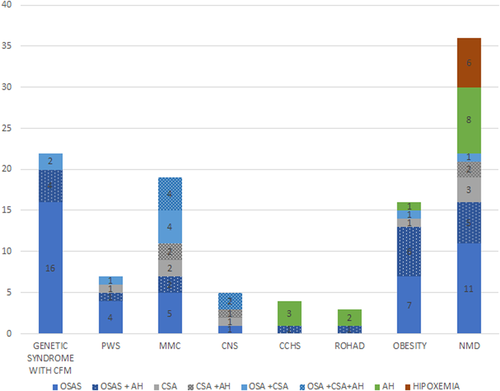
The PSG total population studied (n 165) had a median and interquartile range apnea-hipopnea index (AHI): 21.19/h (6.85-43.43), SaO2 media: 95.80% (93.9-97), SaO2 min: 73.50% (62.25-82.0), total registry time (TRT) less than 90%: 3.50% (0.9-10.60), CO2 media: 48.0 mm Hg (43-53.14), and TRT greater than 50 mm Hg: 44% (4.7-85). Table 3 summarizes PSG results in each SDB group. The OAHI was similar in the OSAS group compared to the mixed-apnea group. The CAI was similar between the CSA group and the mixed-apnea group. Nevertheless, the mixed-apnea group had a higher AHI, spent more time with SaO2 less than 90% and showed a tendency to higher CO2 levels compared to the OSAS and CAI groups. In the H group, hypoxemia was more severe compared to the other groups and the hypoventilation group had the highest CO2 levels. Figure 3 shows SDB groups according to PSG results in the most frequent underlying diseases (n 112). As expected in genetic syndromes with craniofacial malformations (Crouzon, Pfeiffer, mucopolysaccharidoses, Treacher-Collins syndrome, and achondroplasia), Prader-Willi syndrome, and obesity, OSAS was the most frequent SDB found. Pts with MMC, CNS, and NMD showed heterogeneity in their SDB. As expected, all pts with CCHS and ROHHAD evidenced nocturnal alveolar hypoventilation and one patient had associated OSAS in each case. Ventilation was started electively in 116 pts (47.5%), subacutely in 80 (32.8%) and acutely in 48 (19.7%). This took place in our general pediatric ward in 204 pts (83.6%), in the PICU in 20 (8.2%), at the referral hospital in 18 (7.4%), and in the NICU in 2 (0.8%). Noninvasive ventilation (NIV) was the modality more frequently used: 210 (86.1%) pts with NIV vs 34 (13.9%) on invasive mechanical ventilation. Pressure-control mode was used in 182 (74.5%) pts, CPAP was used in 51 (20.9%), bilevel in 9 (3.68%), and hybrid modes (AVAPS) in 2 (0.8%).
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|
| OSAS | CSA | Mixed | Hypoxemia | AH | |
| n 89 | n 16 | n 22 | n 8 | n 16 | |
| OAHI/h | |||||
| Median | 30 | 0 | 31.75 | 0 | 0 |
| IQR | (15-50.3) | (0-2.4) | (13.1-40.1) | (0-0.1) | 0 |
| CAI/h | |||||
| Median | 0.3 | 7.4 | 8.6 | 0 | 0.1 |
| IQR | (0-1.3) | (5.9-12.3) | (5.7-11.8) | (0-0.4) | (0-0.5) |
| AHI/h | |||||
| Median | 31.31 | 8.45 | 38.43 | 0.14 | 0.13 |
| IQR | (15-50.3) | (6.67-15.43) | (27.26-63.14) | (0-1.88) | (0-1.82) |
| SaO2 mean (%) | |||||
| Median | 95.4 | 96.5 | 96.25 | 93.6 | 95.9 |
| IQR | (94-97) | (93.8-97.05) | (93.5-97) | (92.3-94.4) | (93.7-96.4) |
| SaO2 min (%) | |||||
| Median | 69 | 74 | 74 | 79 | 85 |
| IQR | (60.8-78) | (63.2-77.5) | (61.2-78.5) | (77.5-82) | (74.5-89) |
| % TRT < 90% | |||||
| Median | 3.68 | 1.7 | 7.8 | 3.8 | 3 |
| IQR | (0.7-9.6) | (1.1-13.3) | (1.6-12.9) | (2.7-5.8) | (0.2-12.2) |
| Hypoxemia | |||||
| n (%) | 56 (62.9) | 8 (50) | 14 (63.6) | 8 (100) | 9 (56.3) |
| CO2 mean, mm Hg | |||||
| n 50 | n 12 | n 17 | n 5 | n 16 | |
| Median | 46.7 | 46.1 | 49.3 | 39 | 54.25 |
| IQR | (42.3-52.1) | (41.6-50.3) | (44.9-52.6) | (37.6-40) | (49.9-56.5) |
| AH | |||||
| n (%) | 27/50 (54) | 5/12 (41.7) | 10/17 (58.8) | 0/8 (0) | 16/16 (100) |
- Abbreviations: AH, alveolar hypoventilation; AHI, apnea-hipopnea index; CAI, central apnea index; CSA, central sleep apnea; IQR, interquartile range; OAHI, obstructive apnea-hypopnea index; OSAS, obstructive sleep apnea syndrome; PSG, polysomnography; SaO2, oxygen saturation; SDB, sleep disordered breathing; TRT, total registry time.
The current number of pts on follow up is 133/244 (54.5%). A total of 41/244 pts (16.8%) dropped out follow up, 40/244 (16.4%) were transitioned to adult care, 13/244 (5.32%) resolved their SDB, and 13/244 (5.32%) died. All deaths were related to the underlying condition, except for one child who suffered an accidental ventilator disconnection at home. Four pts (1.63%) continued follow up in facilities near their homes.
4 DISCUSSION
This is the first report on a long-term follow-up program of home ventilated children in Argentina. Although this is a single-center report, the “Hospital de Pediatría Prof. J. P. Garrahan” is the national pediatric tertiary care referral center. In fact, in our cohort, one-third of the pts lived more than 500 km away from the hospital; hence, it is a representative sample of children with complex diseases from all regions of our country attended in the public sector. Only, Chilean5-7 and Brazilian authors8, 9 have published reports on other pediatric Latin American experiences on HV. Few pediatric reports have been published from other emerging countries.10-14
The total number of pts admitted to the program, as well as the number of new pts per year, is similar to those reported in the systematic review by Castro-Codesal et al.15 As previously reported worldwide total growth is, on one hand, exponential15-18 and, on the other hand, the number of pts admitted per year presents a bimodal growth, with the rapid increase in the early years to finally achieve a plateau in the last years.15 In our case, this plateau is probably explained partly by the long waiting list for sleep studies. The other factor limiting NIV's start is the lack of bed availability for NIV training.
Community physicians refer pts to the hospital mainly for initial underlying disease diagnosis and treatment. Once a complex disease is confirmed, the patient is referred back to the community physician and, at the same time, followed up at the hospital at intervals according to age, disease, and severity. During the last 11 years, SDB screening in the clinics has increased due to greater awareness, increasing the demand for sleep beds. With continuous education of the pediatric medical community on SDB screening, the demand of sleep studies is expected to grow. This shows, once again, the need for more public sleep beds. Regarding health insurance, 84% of our pts had health insurance, while 16% did not. pts with obesity as the underlying disease do not receive handicapped health insurance. On the other hand, foreign pts have the right to receive free public attention, but they must have lived in Argentina for more than 15 years before turning into candidates to receive handicapped health insurance. The Hospital's Foundation gave temporary financial support for home equipment to 33 pts without health insurance until they had active social security or their local regional administration took over the situation.
Home distance from home to hospital was, in more than half of the pts, greater than 100 km, and in 34% of the cases, more than 500 km. The development of a telemedicine follow-up program would be a key point in the future. As recently stated by Chuo et al,19 telemedicine in complex diseases and home ventilated children need research and development.
More than half of the program population had non-NMD diseases for which ventilation was started. Castro-Codesal et al15 reported that a rising number of children with the central nervous system and cardiorespiratory diseases were started on ventilation in 2011 to 2014 triennium when compared to 2005 to 2008, when most of the pts started on LTHV had NMD. This study is one of the few large cohorts published providing objective sleep study measures on SDB before starting ventilation. A total of 173 (70%) pts had sleep studies performed. Few other centers have published objective sleep measures.15, 20-22 PSG results in our study objectivated similar results as those described by Amaddeo et al21 and an AHI almost double with similar SaO2 values and slightly higher median CO2 than Castro-Codesal.15 In our population, this might be explained partly by the long waiting list to access PSG studies. The more symptomatic and severe pts are given priority in the waiting list, this creates a selection bias that might be responsible for the differences in the PSG results. OSAS was the most frequent SDB found associated to hypoxemia and hypercapnia. Nevertheless, in 41/111 (36.9%) pts with OSAS (including both OSAS and mixed group), the OAHI/h was moderate to severely elevated without hypoxemia, which denotes the importance of performing complete PSG studies in these children with complex diseases. Respiratory polygraphy (RP) underestimates the incidence of hypopneas without hypoxemia.23 No published study has addressed nor validated the use of RP in children with complex diseases. Children with NMD disease had OSAS as well as hypoventilation or hypoxemia demonstrated in their sleep studies. This bimodal SDB distribution has already been mentioned in the literature and explained by age differences and motor compromise.24 The fact that many pts with NMD were started on ventilation without sleep studies, on the basis of their clinical status, might explain the lower than expected incidence of objectively documented hypoventilation in these pts.
Sleep studies are important for defining in many cases the need for ventilation. In our country, access to complete sleep studies (level 1) is limited in the public sector. Our hospital is the only pediatric public hospital with two available fully equipped sleep beds to study SDB in complex diseases. Considering the severity of SDB in our cohort, public policies need to be developed to increase the number of available pediatric sleep beds in the public sector given that delay in diagnosis can be deleterious.
All pts were started on ventilation as in-pts, most of them (83%) in the general pediatric ward. Two groups have published outpatient long-term ventilation initiation experience and compared them to in patient experience with promising results.25, 26 As stated before, lack of bed availability for family NIV training explains the delay in ventilation start. In the future, an outpatient training program could overcome this limitation. Recently, we have worked in an outpatient modality in a few families, due to different reasons, such as lack of bed availability, increased risk of intrahospital respiratory infections in the winter season, absence of severe hypoxemia, with promising results. This modality needs to be explored in a protocolized manner to compare results objectively between the inpatient and the outpatient modality. Once again, considering the severity of SDB in these children with complex diseases, as clearly shown in our work in Table 3, public policies are needed to develop outpatient training programs.
Noninvasive interfaces were far more frequent than invasive (86.1% vs 13.9%). Worldwide, this has shown to be the progressive trend.17, 27
We included pts treated with CPAP in this cohort even though it does not provide a true augmented ventilation. This is due, on one hand, to the fact that the program centralizes the follow up of all pts either on CPAP or true ventilation, and on the other hand, because other authors have already considered them altogether.16, 28, 29
Regarding follow up, 54% of the pts are still on HV and on follow up, resembling other published cohorts.15, 18 Forty (16.4%) pts were successfully transitioned to adult care. As clearly expressed by Chatwin et al,16 the increased survival of children with chronic ventilatory failure has serious implications for the need for planning transition to adult care programs.
Study limitations: this is a single-center report and, although results might not be generalizable to all Argentine centers, it is a representative sample of pts living along the country attending a public health hospital.
Outcome measures like compliance with ventilation was not evaluated for the purpose of this study (we only evaluated adherence to follow up, ie, adherence to the programmed visits in our hospital) because during the first years of the program, not all the HV machines had memory cards or intelligent systems to download adherence data and objectively record compliance. This is an interesting topic to address in the future.
Other outcome measures like comparative PSG results after ventilation initiation was not addressed and need to be studied in the future.
Cost of care was not analyzed. This is undoubtedly an interesting issue for the future. A cost-effectiveness study could support the need for a health budget allocated to pediatric HV in our country.
5 CONCLUSION
This is the first Argentine pediatric report on long-term follow up on HV. As worldwide, our results reflect the increase in the number of pts needing chronic HV.
The outstanding characteristics of our follow-up program is that it takes care of pts along the whole country and that a great effort has been done to combine clinical and objective measures to better decide when to start ventilation.
This long-term experience underlines the feasibility of a HVP in an emergent country.
ACKNOWLEDGMENTS
The authors would like to thank Prof Anita Simonds for the manuscript revision, suggestions, and encouragement and Jorgelina Taveira, Medical Editor, for the final revision. They would also like to thank the HVP team Diego Amoedo, MD (pediatrician); Marisa Armeno, MD (Department of Clinical Nutrition); Silvina Borettini, TECH (Sleep Unit); Valeria Greif, MD (Psychiatrist); Carola Saure, MD (Department of Clinical Nutrition), At last they would like to thank the general ward pediatricians involved with the HVP, the Neuromuscular team, the Physical Therapy Department team, the Respiratory Endoscopy Department team, and Manuela Dicembrino, MD (Sleep Unit research fellow 2016-2017), for their invaluable daily work.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.




