Review article: sarcopenia in cirrhosis – aetiology, implications and potential therapeutic interventions
Summary
Background
Sarcopenia (loss of muscle mass) is common in cirrhosis and is associated with poor outcomes. Current teaching recommends the use of protein supplementation and exercise, however, this fails to address many other factors which contribute to muscle loss in this setting.
Aims
To summarise existing knowledge regarding the aetiology of sarcopenia in cirrhosis, diagnostic modalities and the clinical significance of this condition. In addition to discuss recent research findings that may allow the development of more effective treatments.
Methods
We conducted a Medline and PubMed search using the search terms ‘sarcopenia’, ‘muscle’, ‘body composition’, ‘cirrhosis’, ‘liver’ and ‘malnutrition’ from inception to October 2015.
Results
Cirrhotic patients with sarcopenia have reduced survival, experience increased rates of infection and have worse outcomes following liver transplantation. The aetiology of this condition is more complex than simple protein and calorie malnutrition. Cirrhosis also results in depleted glycogen stores and metabolic alterations that cause excessive protein catabolism, increased activation of the ubiquitin–proteasome pathway and inappropriate muscle autophagy. Satellite cell differentiation and proliferation is also reduced due to a combination of elevated myostatin levels, reduced IGF-1 and hypogonadism. Although there is some evidence supporting the use of late evening snacks, branched chain amino acid supplementation and high protein/high calorie diets, well designed clinical trials addressing the effects of treatment on body composition in cirrhosis are lacking.
Conclusion
Sarcopenia in cirrhosis has a complex pathogenesis and simple dietary interventions are insufficient. Improved understanding of the multiple mechanisms involved should allow the development of more effective therapies, which target the specific underlying metabolic derangements.
Introduction
Loss of muscle mass and function, or sarcopenia, is one of the commonest features of cirrhosis and contributes significantly to morbidity and mortality in this population. Its prevalence in patients with cirrhosis is estimated to be 40–70% and thus it currently affects over 300 000 people in the USA.1-4 To put this into context, the annual incidence of bleeding oesophageal varices in cirrhosis is 10–15%, refractory ascites is 5–10% and hepatocellular carcinoma occurs in 3–5%.5 Only overt hepatic encephalopathy has similar prevalence, and interestingly has a close correlation with the presence of sarcopenia.6, 7 The prevalence of sarcopenia in cirrhosis is far higher than in other gastrointestinal illness due to the unique metabolic derangements caused by liver failure. The prevalence in inflammatory bowel disease for example is only 21%.8
Despite this, there are no established criteria for the diagnosis of this important complication of cirrhosis, its pathogenesis is poorly understood and none of the proposed treatment options have been well explored in randomised clinical trials. This review aims to summarise current understanding of the pathogenesis of sarcopenia in patients with cirrhosis, discusses possible treatment options and highlights potential avenues for future research.
We conducted a Medline and PubMed search from inception to October 2015 using the search terms ‘sarcopenia’, ‘muscle’, ‘body composition’, ‘cirrhosis’, ‘liver’ and ‘malnutrition’. This was followed by a manual review of the literature to select articles that specifically addressed the diagnosis, aetiology, implication or treatment of sarcopenia in cirrhosis.
Normal regulation of muscle mass in adults
The regulation of muscle mass and function in adults is tightly controlled by various genes and transcription factors that affect protein production, recruitment and differentiation of satellite cells and muscle breakdown. These pathways are summarised in Figure 1.

Muscle protein synthesis
Activation of the intracellular target mTOR (mammalian target of rapamycin) stimulates protein synthesis via various intracellular signalling pathways. mTOR is activated via protein kinase B (PCK/AKT), which is upregulated by insulin, insulin-like growth factor (IGF-1), circulating testosterone, physical exercise and branched chain amino acids (BCAAs), particularly leucine.9
Satellite cell differentiation and proliferation
Muscle growth and repair also requires the recruitment and proliferation of satellite cells, which are the precursors to new muscle fibres. Protein kinase B (PCK/AKT), which is directly activated by IGF-1, stimulates this activation of satellite cells. This pathway is upregulated by BCAAs, exercise and testosterone.10, 11
Myostatin is the major negative regulator of satellite cell differentiation and proliferation. It belongs to the transforming growth factor-β superfamily of secreted growth and differentiation factors. It acts in a paracrine fashion to maintain satellite cells in a quiescent state within muscle. IGF-1 has dual action in that it stimulates mTOR as well as inhibits myostatin, thereby stimulating muscle growth by both activating satellite cells and muscle protein synthesis.12
Proteolysis
The two major skeletal muscle proteolytic pathways are the ubiquitin–proteasome pathway (UPP) and the autophagy system. The UPP is the major muscle breakdown pathway, and involves the tagging of protein by conjugation with ubiquitin and then subsequent degradation by the 26S proteasome. Protein kinase B (PCK/AKT) inhibits proteolysis via the UPP and both inactivity and systemic inflammation activate the UPP. Autophagy is a programmed cell death pathway activated in response to cell stress of any aetiology that involves the fusing of a cell to a lysosome which then degrades cellular contents.13
Aetiology of sarcopenia in cirrhosis
There are multiple factors which may contribute to sarcopenia in cirrhosis, although their precise roles are poorly understood (Figure 2). Ultimately, this condition results from an imbalance between muscle formation and muscle breakdown, although the specific pathways involved may differ between patients. Understanding the role of these potential contributors will help provide a rational basis for developing strategies to prevent and treat muscle loss in patients with advanced cirrhosis and to minimise its impact on patient quality of life, morbidity and mortality.
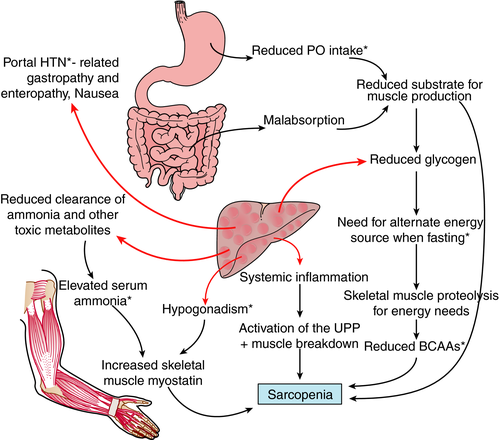
Malnutrition – reduced substrate for muscle production
Malnutrition is believed to play a major role in sarcopenia in cirrhosis. A reduced total energy intake has been linked with a higher prevalence of sarcopenia in patients with cirrhosis. For example, one observational study of 50 patients reported a median caloric intake of 1544 kcal in sarcopenic patients compared to 1783 kcal in nonsarcopenic patients (P = 0.001).14 However, this does not necessarily prove causality since lower caloric intake may more broadly reflect general ill health. There are many potential causes of reduced dietary intake in cirrhotics including nausea and anorexia induced by elevated inflammatory mediators such as TNF-alpha; increased intra-abdominal pressure from ascites; abdominal pain and altered gut motility.15 Dysgeusia is common in cirrhosis, and salt restriction may also affect enjoyment of food, both of which also may affect caloric intake.16 Patients may also have inadequate dietary protein intake due to inappropriate and outdated advice that protein restriction is required to prevent encephalopathy.
Even with adequate caloric intake, malabsorption may contribute to net negative energy balance in cirrhosis. It is well recognised that reduced bile flow can cause malabsorption of fats and fat-soluble vitamins including vitamin D.17 There is also increasing evidence that altered gut motility, small bowel bacterial overgrowth and changes to the gut microbiota in cirrhosis can each affect both absorption and utilisation of nutrients.18 Alcohol abuse may result in concomitant pancreatic insufficiency, which can also impair nutrient absorption.
Elevated myostatin and inhibition of muscle growth
Myostatin is the predominant negative regulator of satellite cell differentiation and proliferation. Elevated myostatin levels are thought to be one of the major driving forces underlying the sarcopenia of ageing, however, the reasons for this elevation are not fully understood.19 Blocking myostatin increases muscle strength and function in animal models of sarcopenia of ageing20 which further strengthens its aetiological role. Patients with cirrhosis have been shown to have significantly higher serum levels of myostatin than controls (0.53 μg/mL vs. 0.13 μg/mL in cirrhotics and controls respectively, P = 0.002).21 Muscle biopsies in cirrhotics have also demonstrated significantly increased myostatin expression compared to controls.22
Although elevated myostatin levels may make an important contribution to sarcopenia in cirrhosis, the cause is uncertain. One possible mechanism is that ammonia levels are frequently elevated in cirrhosis, and that this increases myostatin expression. In a murine cirrhotic model, ammonia (administered via intraperitoneal injection) was shown to stimulate myostatin expression in muscle via the activation of the transcription factor nuclear factor kappa beta.23 Furthermore in this model, muscle wasting was induced by ammonia in wild-type but not myostatin knock-out mice and there was a positive correlation between serum ammonia and myostatin expression.
The reduction in serum testosterone levels and IGF-1 levels in cirrhosis24, 25 likely also contribute to elevated myostatin levels since these mediators normally act to suppress myostatin expression.26, 27 The possible relationship between myostatin and other stimulators of muscle differentiation is represented in Figure 1.
Altered metabolism – abnormal use of protein as an energy source
The metabolic alterations in cirrhosis are far more complex than simple malnutrition and reduction in muscle protein production. Cirrhosis mimics the state of starvation, with inappropriate use of body fat and protein stores for gluconeogenesis. This proteolysis and lipolysis can occur even during short periods of fasting such as overnight, which should not happen under normal physiological conditions. This switch to the use of fat and protein as an energy source is thought to due to a reduction in hepatic glycogen stores which results in a need to generate glucose from alternate sources.28
One study demonstrated that energy use derived from carbohydrate during a 12 h overnight fast was only 13% in cirrhotics compared to 39% in normal subjects.29 In controls at least 36 h of fasting was required for the proportion of calorie production from fat and protein to approach that in overnight fasted cirrhotics. Several other studies have documented increased ketogenesis and amino acid consumption in cirrhosis5, 30, 31 and respiratory quotient (carbon dioxide production as compared to oxygen consumption) has been shown to be reduced in cirrhosis (0.63 ± 0.05 vs. 0.84 ± 0.06 in controls, P < 0.001), which again reflects a lower proportion of energy derived from carbohydrate.32
In keeping with these findings, whole body protein turnover was found to be increased from 1.8 ± 0.3 g of lean body mass per 9 h to 3.14 ± 1.2 g of lean body mass per 9 h in cirrhotics.30 The consumption of body fat and protein stores in cirrhotics is likely further exacerbated by an overall increase in resting energy expenditure, in part driven by the chronic upregulation of inflammatory mediators.33 This metabolic switch to the use of amino acids to create glucose results in reduced levels of circulating branched chain amino acids (BCAAs) in patients with cirrhosis.34 This may further compounds muscle breakdown since BCAAs are the preferential energy source of skeletal muscle and muscle tissue.35
Relationship between hepatic encephalopathy and muscle mass
A recent prospective study identified a significant correlation between the presence of hepatic encephalopathy and sarcopenia in cirrhosis. The prevalence of sarcopenia was found to be 30% in patients without encephalopathy, 49% with minimal change encephalopathy and 56% with overt encephalopathy.36 This may reflect a causative relationship between these two common sequelae of cirrhosis.7 Although BCAAs are utilised for energy production in cirrhosis, the main cause for reduced BCCA levels is their uptake by muscle to assist in ammonia detoxification via glutamine synthase.37 Muscle uptake of BCAAs is significantly higher in cirrhotics than controls (32 vs. −12 μmol/L blood) and leads to a reduced BCAA/aromatic amino acid ratio (1.65 vs. 2.73, P < 0.05).34 Thus in sarcopenic cirrhotic patients, both reduced circulating BCAA levels and reduced muscle mass may contribute to impaired ammonia clearance. A Cochrane review has concluded the BCAA therapy has a beneficial effect on hepatic encephalopathy (RR 0.76, 95% CI 0.63–0.92) which reflects the important role BCAAs play in ammonia clearance38 but few studies investigate the impact of BCAAs on muscle mass.
Activation of the UPP and autophagy – increased muscle breakdown
A review of 21 studies examining sarcopenia of ageing concluded that a strong link exists between inflammation and sarcopenia, with TNF-alpha and interleukin-6 most commonly implicated, with some of these studies reporting muscle biopsy data that suggest the mechanism is related to oxidative stress.39 Cirrhosis is a pro-inflammatory state, in which levels of TNF-alpha and other inflammatory mediators such as interleukin-1 and interleukin-6 are elevated.40 In experimental cirrhosis a strong correlation was demonstrated between muscle TNF-alpha and muscle ubiquitin levels (r = 0.86, P < 0.001), which reflects the activation of the UPP, suggesting that in cirrhosis, inflammation may also contribute to sarcopenia.41 A recent murine study showed that ethanol can stimulate muscle autophagy without altering proteasome activity or the UPP,13 indicating that alcohol induced autophagy may directly contribute to sarcopenia in patients with alcoholic liver disease.
Hormone deficiency
In men, testosterone levels correlate with muscle mass across low to supraphysiological levels.42 This hormone has dual positive effects in muscle, by both inhibiting myostatin production26 which leads to less inhibition of satellite cell activity, and increasing IGF-1 levels,43 mTOR activation and muscle protein synthesis.43 Up to 90% of men with cirrhosis have low testosterone levels, due to defects at all levels of the hypothalamic–pituitary–testicular axis.44, 45 In addition, SHBG is often elevated, which strongly binds testosterone and can thereby lead to an even greater reduction in free testosterone.25 The possibility that low testosterone levels contribute to sarcopenia in men with cirrhosis is supported by a recent study which demonstrated a correlation between testosterone levels, sarcopenia and overall mortality (P = 0.019).4
IGF-1 levels are reported to be low in cirrhosis24 which would be expected to result in reduced mTOR-mediated activation of muscle protein synthesis and increased myostatin levels.27 Although a relationship has been established between falling IGF-1 and the sarcopenia of ageing,46 a similar relationship has not been established in cirrhosis. In one study, no correlation between IGF-1 and muscle mass was found in a cohort of 64 hospitalised cirrhotics, however, this may be due to the effect of acute illness on IFG-1 levels.47 Thus, although a link between reduced IGF-1 levels in cirrhosis and sarcopenia is scientifically plausible, this requires further study.
Diagnosis of sarcopenia
Clinical features and functional measures
Anthropometry
Body mass index can be a poor indicator of nutritional status in patients with cirrhosis as it can be falsely elevated due to fluid overload in the context of portal hypertension48 and also cannot differentiate fat mass from muscle mass. Therefore, we do not recommend the use of such techniques.
Hand grip strength
Hand grip strength is an objective measure of functional muscle strength of the hand and forearm. This conveys important information that could potentially translate to the capacity for physical activity which is not necessarily captured by muscle mass quantification. It correlates modestly with muscle thickness of the hand and forearm (correl 0.379, P = 0.001 in men and correl 0.268, P = 0.002 in women).49 It is interesting to note that some studies, particularly in the elderly, show that it is possible to stimulate an increase in muscle mass without necessarily demonstrating an increase in strength or physical activity.50 Hand grip strength has been shown in a large population study of >140 000 healthy adults to be independently correlated with all-cause mortality (hazard ratio per 5 kg reduction in grip strength of 1.16 (CI 1.13–1.20), P < 0.0001.51
Frailty
In the general population functional measures of frailty are powerful predictors of mortality. For example, in a study of elderly patients, failure to complete the 6 min walk test, was the best predictor of mortality with a hazard ratio for mortality of 3.26 (CI 1.38–7.69).52 The advantages of such measures are their ability to be repeated serially in out-patient settings as well as their non-invasiveness. Limitations include subjective components and difficulty in standardisation.
Measures of frailty derived from geriatric populations have been applied to patients on the waitlist for liver transplantation, where they have also been shown to have prognostic value. These include the 6 min walk test (frail defined as <250 m), the Fried Frailty Index (FFI) (frail defined as ≥3 out of 5 points) and the short physical performance battery (SPPB) (frail defined as a SPPB score of <9 out of 15).53, 54
Quantitative investigations
Low serum albumin may be an indicator of protein malnutrition but can also be reduced in the context of cirrhosis due to hepatic synthetic dysfunction. Older quantitative investigative techniques used to assess muscle mass included urinary creatinine excretion or underwater weighing which have suboptimal accuracy and provide limited information. Other more experimental techniques include neutron activation analysis and air displacement plethysmography which are highly accurate but not practical in the clinical setting.55 There are, however, techniques that can be performed within a normal hospital setting using easily accessible methods.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) provides high-resolution three-dimensional images which can accurately differentiate tissue planes. Volume calculations can subsequently be performed to create a complete body composition model for the entire body in a single scan. MRI has been used for research purposes to accurately display differences in muscle composition between men and women, and loss of lower limb muscle mass with ageing. It is thought to be highly accurate and no ionising radiation exposure is required.56 Limitations of this technique include high cost and limited access and this has precluded its use in routine clinical practice.
Computerised tomography
Computerised tomography (CT) is also able to differentiate muscle from other body tissues, and create a three dimensional model similar to MRI. It is quick, relatively cheap, readily available and provides a high degree of accuracy. However, whole body CT scanning exposes patients to unacceptably high ionising radiation levels which have been linked to increased rates of subsequent malignancy.57, 58 Therefore, whole body CT is not an acceptable tool for routine and repeated quantification of body composition.
Assessment of thickness of the psoas muscle on CT scan has been employed as an estimate of muscle mass.59 However, skeletal muscle area derived from a single slice CT has become more easily reproducible, and reduces the radiation exposure required to only 2.6 millisieverts. Measuring cross-sectional muscle area at either the level of the third (L3) or fourth (L4) lumbar vertebrae has been shown to correlate well with total body muscle mass (r = 0.71), and it is now accepted as a safe and accurate means of estimating muscle mass.60 When adjusted for patient height to take stature into account it is referred to as skeletal muscle index.61
L3 skeletal muscle index has been shown to be of higher accuracy in the diagnosis of sarcopenia in cirrhosis than anthropometry or DEXA scanning62 and is now the most commonly employed method in studies investigating sarcopenia in cirrhosis.61, 63 Diagnostic criteria have been extrapolated from oncology populations, where sarcopenia is defined as <38.5 cm/m2 for women and <52.4 cm/m2 for men.64 Multiple studies in cirrhosis which have employed this definition have produced clinically meaningful results and it is increasingly accepted as the most appropriate definition of sarcopenia when using cross-sectional imaging.3, 61, 65, 66
Dual-energy X-ray absorptiometry
Dual-energy X-ray absorptiometry (DEXA) scanning uses multiple low-dose X-rays to create a three-dimensional compartmental model which is highly accurate at differentiating fat, fat-free mass and bone mineral mass. It has been shown to correlate well with gold standard experimental techniques such as neutron activation analysis.55 A frequently used measure to assess functional muscle is appendicular lean mass (APLM), which specifically quantifies arm and leg lean mass. APLM has been shown to correlate well with total body muscle mass as measured by whole body MRI.67 Sarcopenia has been defined as an APLM index (APLM/height2) of ≥2 standard deviations below the mean, which for a caucasian 40-year old corresponds to an APLM index of <4.61 kg/m2 in women and <6.57 kg/m2 in men.68
Dual-energy X-ray absorptiometry has been evaluated in patients with cirrhosis of varying aetiologies and severities. Neutron activation analysis was again used as a gold standard, and DEXA was shown to provide accurate estimations of fat-free mass and fat mass.69 The APLM skeletal muscle index has also been employed to examine contributing factors to sarcopenia in cirrhotic patients.14 The major limitation of DEXA is its inability to differentiate water from muscle. As a result, oedematous tissue can falsely elevate muscle mass readings70 which is a potential concern in cirrhotics though confounding by ascites is removed by the use of APLM.37
A major benefit of DEXA is that it is cheap and readily available. In addition, the very low dose of radiation per examination makes it safe to be used for serial measurements. Given its safety, proven accuracy and reproducibility, we believe DEXA-measured APLM is likely to be the most appropriate diagnostic tool to monitor response to intervention in future trials in cirrhosis.
Implications
Pre-transplant mortality
The prognostic importance of sarcopenia in cirrhosis is becoming well recognised with multiple reports that it is associated with mortality independent of the MELD score, the tool most widely used to predict prognosis in patients awaiting liver transplantation.3, 4, 59, 61, 63, 66 A summary of some of the key studies investigating the impact of sarcopenia on mortality are represented in Table 1.
| Study type | n | Method for measuring muscle | %Sarcopenic/frail | Mortality riska |
|---|---|---|---|---|
| Quantitative59 | 376 | Transverse psoas muscle thickness/height (TPMT/height) by CT | nd | 15% increase in mortality for each unit decrease in TPMT/height independent of MELD score |
| Quantitative63 | 89 | TPMT by CT. Sarcopenia defined as <14 mm/m | 31 | HR 5.398 for mortality on multivariable analysis if sarcopenia present |
| Quantitative61 | 112 | L3 skeletal muscle index by CT (SMI = muscle area/height2), sarcopenia defined as <38.5 cm/m2 for women, <52.4 cm/m2 for men | 40 | HR 2.26 for mortality on multivariable analysis if sarcopenia present |
| Quantitative66 | 142 | L3 SMI by CT, sarcopenia defined as <38.5 cm/m2 for women, <52.4 cm/m2 for men | 41 | HR 2.36 for mortality on multivariable analysis if sarcopenia present |
| Quantitative65 | L3–4 SMI by CT, sarcopenia defined as <38.5 cm/m2 for women, <52.4 cm/m2 for men | 68 | HR 0.95 for post-transplant mortality for each unit increase in SMI in men only (no significant impact in women) | |
| Quantitative72 | 130 | L3 SMI by CT, sarcopenia defined as <38.5 cm/m2 for women, <52.4 cm/m2 for men | 68 | HR 3.03 for mortality on multivariable analysis if sarcopenia present |
| Quantitative72 | 145 | L4 SMI by CT, sarcopenia defined as <52.4 cm/m2 (men only) | 70.3 | HR 1.05 for mortality on multivariable analysis for each unit decrease in SMI |
| Functional53 | 121 | 6-min walk test, frail defined as <250 metres | nd | Each 100 m reduction in 6-min walk test associated with reduced survival (HR 0.48) independent of MELD score |
| Functional54 | 294 |
Fried Frailty Index, frail defined as ≥3 out of a maximum of 5 points Short physical performance battery, frail defined as <9 of maximum 12 points |
17 31 |
45% increase in mortality with each 1 point increase in FFI, independent of MELD 19% increase in mortality with each 1 point decrease in SPPB, independent of MELD score |
| Functional71 | 50 | Hand grip strength, frail defined as two standard deviations below the mean | 63 | 20.7% 12 month mortality in frail patients vs. 0% in nonfrail patients |
- a All mortality results displayed were considered significant with a P < 0.05.
As in geriatric populations, functional measures of frailty have been associated with survival in cirrhosis, independent of the MELD score. Each 100 m reduction in the 6 min walk test in one cohort of cirrhotics was independently associated with reduced survival (HR 0.48).53 In a large pre-transplant cohort, each increase by one point in the FFI score was associated with 45% increase in mortality, and each reduction by one point in the SPPB score correlated with a 19% increase in mortality.54 Reduced grip strength (≥2 s.d. below the mean) has been associated with 20.7% 12 month mortality as compared to 0% mortality in those with higher readings (P < 0.05).71
Mortality has been demonstrated to be from 2.3 to 5.4 times higher in patients meeting diagnostic criteria for sarcopenia as compared to those without, independent of the MELD score (Table 1). In addition, there is evidence that a rapid rate of decline in muscle mass predicts mortality, with a hazard ratio for mortality on multivariable analysis of 2.73 (95% CI 1.43–5.44) in patients with >3.1% change in skeletal muscle index per year as compared to less dramatic muscle loss.72
The utility of sarcopenia as a predictor of outcomes has been assessed by the incorporation of L3 skeletal muscle index on CT scan into a MELD score model to assess prognosis in a study in of 669 cirrhotic patients. The MELD-sarcopenia model had improved predictive value for 12 month mortality compared to the MELD score alone (c statistic 0.77 vs. 0.73, P = 0.03) and was particularly useful in patients with MELD score <15 (c statistic 0.85 vs. 0.69, P = 0.02).73 Validation is required in other studies. This should include investigation of the post-transplant outcomes of sarcopenic patients since this may facilitate allocation of organs to patients who will gain most benefit, and help identify a sarcopenic threshold below which transplantation is likely to be futile.
Although sarcopenia is associated with increased mortality, there is evidence to suggest that its reversal improves survival. In a study of TIPSS outcomes, muscle mass improved post-TIPSS in 41 of 57 patients. The 12-month mortality of patients with reversal of sarcopenia was 9.8% vs. 43.5% in those in whom sarcopenia persisted (P = 0.007).74 This highlights the importance of identifying strategies for improving muscle mass in this patient group.
Infection risk
In both pre- and post-transplant populations, sarcopenia has been associated with increased infection risk. An early study employing L3 SMI to quantify muscle mass showed that the increase in mortality associated with sarcopenia was attributable to an increase in sepsis-related deaths.61 An earlier study investigating the impact of mid-arm circumference demonstrated increased infection risk in those below the 25th percentile as compared to those above (32% vs. 8%, P = 0.02).75
In the post-liver transplant setting, infection risk was found to be fourfold higher in patients with the lowest quartile of muscle mass compared with the highest quartile.76 Sarcopenia in the elderly has also been associated with increased infection risk (RR 2.1).77 This may explain the association between sarcopenia and mortality in cirrhosis, given this population is already at particularly high risk of sepsis-related death.78
Post-transplant outcome
Sarcopenia has also been linked with poorer post-transplant outcomes, including increased length of stay and reduced survival, and thus prioritisation of patients on the basis of sarcopenia may not be an appropriate strategy. In male transplant recipients, each 10% incremental increase in muscle mass was associated with a 9% shorter length of stay, and a 12% shorter ICU stay. Each one unit decrease in L3 SMI resulted in a 5% increase in risk of death.65
Quality of life
In addition to its effects on mortality, sarcopenia likely contributes to reduced quality of life in cirrhosis. Muscle weakness limits exercise capability, can profoundly impact simple activities of daily living and may also contribute to fatigue. These effects can severely impact participation in the community, family and workforce. Altered muscle physiology is also thought to contribute to muscle cramps which are common in cirrhosis and have been identified as a major factor affecting quality of life.79, 80
Metabolic function
Sarcopenia itself can also lead to secondary alterations in metabolic function that may further contribute to the progression of liver disease. Muscle is a highly metabolically active tissue that contributes significantly to insulin-mediated glucose uptake.81 As a result, sarcopenia reduces glucose uptake and increases insulin resistance, which has been associated with accelerated progression of liver fibrosis and the development of hepatocellular carcinoma.82, 83 Healthy muscle also secretes biologically active mediators known as myokines, which have a range of effects including mediation of inflammation.84 Sarcopenia may therefore potentiate the pro-inflammatory state of cirrhosis which itself can lead to further reductions in muscle mass.
Significance in men vs. women
Data in both pre- and post-transplant populations have suggested the prognostic impact of sarcopenia in cirrhosis is more marked in men.61, 65 The reason for the stronger association in men has not been studied, but it could potentially relate to a reduction in testosterone levels.
Potential therapeutic interventions
Potential treatment options, mechanisms of action and supporting evidence are presented in Table 2.
| Intervention | Administration | Mechanism | Evidence |
|---|---|---|---|
| High energy, high protein diet | 1–1.5 g of protein per kg of body weight per day (total 35–40 kcal/kg total energy intake per day | Improves nitrogen balance | 4.3 ± 3.2 g of nitrogen/day on a high energy, high protein diet vs. −2.2 ± 1.9 g/day on usual diet, P = 0.0130 |
| Late evening snack | Complex carbohydrate snack with protein such as cheese on wholemeal bread | Minimises muscle breakdown during overnight fasting in the context of low hepatic glycogen stores | Metanalysis revealed increased respiratory quotient (increased use of glucose) and nitrogen balance and reduced muscle protein breakdown22, 28, 74, 93 |
| BCAA supplementation | Oral granules leucine/isoleucine/valine 7.5 g/3.75 g/3.75 g (dissolved in carbonated beverage) | Activate muscle protein synthesis via mTOR signalling pathway | BCAA reduced whole body protein Ra (representing reduced proteolysis) and increased GCN2 (muscle regulator of mTOR activity), P < 0.0122, 28, 74, 93 |
| Exercise | We recommend 30 min of moderate intensity walking 3–4 times per week in combination with resistance training such as light hand weights three times per week as tolerated | Activates IGF-1 which results in increased mTOR signalling and downregulation of muscle breakdown via the UPP | Nil. But strongly recommended |
| Testosterone therapy | Intramuscular testosterone decanoate or topical testosterone gel as per manufacturers recommendations | Activates androgen receptors in muscle which inhibits myostatin, activates muscle protein synthesis via the mTOR pathway and downregulates the UPP | Testosterone gel in 12 hypogonadal men with cirrhosis increased hand grip strength from 34.03 ± 7.24 kg to 39.18 ± 5.99 kg, P < 0.001, nb no control group91 |
| Normalisation of portal HTN | Insertion of trans-jugular intrahepatic porto-systemic shunt | Unknown. May improve nutrient absorption by reducing gastropathy/enteropathy, and may reduce systemic inflammatory driver of muscle breakdown | Psoas muscle area improved in 41 of 57 patients with a mean increase from 22.8 ± 0.9 to 25.1 ± 0.9 cm2, P < 0.00174 |
| Rifaximin | 550 mg twice daily | Hypothetically may downregulate myostatin levels by reducing serum ammonia which may also release more BCAAs for use as muscle fuel | Nil. Theoretical only |
|
IGF-1 therapy Myostatin blockers |
Research use only | Increases mTOR signalling. Reduces myostatin-induced blockage of satellite cell differentiation and proliferation | No human studies |
Diet
Simply increasing caloric intake does not prevent the development of sarcopenia in cirrhosis.85 However, there is evidence to suggest that a high-energy, high-protein (HEHP) diet can improve nitrogen retention (4.3 ± 3.2 g of nitrogen/day on HEHP as compared to −2.2 ± 1.9 g of nitrogen/day when fasting, P < 0.01).30 A late evening snack has also been shown to improve nitrogen balance in end-stage cirrhosis compared to usual diet (5.53 ± 0.53 g/day vs. 3.57 ± 0.78 g/day, P < 0.01), which acts to minimise the exaggerated starvation response that occurs during overnight fasting.28 Dietary interventions should therefore routinely incorporate adequate protein intake and the consumption of a long-acting energy source including complex carbohydrates in the late evening.
Given that BCAA levels are low in cirrhosis, the potential therapeutic role of leucine supplementation has been investigated. This theoretically provides an energy substrate for muscle as well as stimulating muscle protein synthesis via activation of the mTOR pathway. One small trial demonstrated significantly reduced autophagy and improved mTOR signalling in human muscle following administration of leucine, and this was associated with increased muscle protein synthesis.22 Activation of the mTOR pathway was also increased by BCAA administration in an animal model of cirrhosis.11 A recent Cochrane review concluded that BCAA administration improves clinically apparent hepatic encephalopathy,38 however, randomised controlled trials of BCAA therapy specifically assessing muscle mass in cirrhotic subjects are lacking.
Exercise
Although there is evidence suggesting that lack of physical activity is associated with sarcopenia and worse outcomes in patients with cirrhosis,86 there is no specific evidence that physical therapy can reverse sarcopenia in this setting. However, it seems reasonable to suggest that gentle physical therapy may help prevent further muscle loss and maintain physical function. It is still unclear if resistance or cardiovascular training is most effective, but in studies in sarcopenia of ageing, any physical exercise programme has benefit compared to placebo.87 It should be noted that the nutritional effects of physical therapy need to be monitored given that increased energy expenditure may accelerate protein catabolism. If tolerated, we recommend walking 30–40 min three to four times per week, and lifting light weights such as hand weights two to three times per week.
Drug therapies
Given the known alteration in myostatin levels in cirrhosis, myostatin blockers have been proposed as a potential treatment for sarcopenia in this setting. A recent phase 2 trial in frail older patients has reported that a humanised monoclonal anti-myostatin antibody increases APLM [+0.43 kg (CI 0.19–0.66) on active treatment, P < 0.001] as well as increases gait speed (+0.05 m/s, P = 0.088)88 but there are no human trials in cirrhotics.
IGF-1 has been shown to improve nitrogen retention in cirrhotic rats,89 as well as reduce myostatin and increase muscle mass90, however, randomised trials in humans have not yet been performed. The effects of testosterone therapy on muscle have not been extensively evaluated in men with cirrhosis, however, one small uncontrolled trial showed an improvement in hand grip strength with topical testosterone therapy (34.0 kg at baseline as compared to 39.2 kg post-treatment, P < 0.001).91
Therapies used to treat hepatic encephalopathy such as lactulose and rifaximin reduce ammonia levels. Given that ammonia consumes BCAAs in muscle as well as activates myostatin, these therapies could potentially have beneficial effects on muscle mass, but this has not been investigated.
Reversal of portal HTN
As outlined above, one uncontrolled study demonstrated an increase in muscle mass and an associated improvement in prognosis following successful TIPSS placement. Psoas muscle area improved in 70% of patients with an increase in the mean area from 22.8 cm2 at baseline to 25.1 cm2 post-TIPSS (P < 0.001).74 This improvement could relate to a reduction in portal pressure, however, many other factors such as reduced ascites, reduced metabolic rate, increased appetite and improved nutrition could have contributed to this effect. This intriguing finding requires validation in a well-designed randomised controlled trial.
Liver transplantation
Liver transplantation is the definitive treatment for advanced cirrhosis and its complications. Few studies have specifically investigated the effect of transplantation on muscle mass and the available data are conflicting. Many of the contributors to sarcopenia are removed by transplantation, with the restoration of normal hepatocyte function and portal pressure, however, immunosuppressive medications such as corticosteroids, calcineurin inhibitors and mTOR inhibitors are all known to adversely affect muscle mass. Published data suggest that muscle mass can stabilise, increase or decrease after liver transplantation.92, 93 Clearly further investigation and longer term studies are required to better clarify this issue.
Conclusions
Sarcopenia is one of the most commonly encountered features of cirrhosis and is associated with significant morbidity and mortality. The classic teaching that this condition responds to a high protein diet and exercise ignores the complex metabolic alterations in cirrhosis that contribute to muscle loss. With increasing understanding of the pathogenesis of sarcopenia in this population, we are moving closer to developing treatments that targets specific changes in energy requirements, metabolic pathways, hormonal abnormalities and nutritional deficiencies.
Authorship
Guarantor of the article: Peter W Angus.
Author contributions: Dr M. Sinclair contributed towards study design, research/literature collation and writing of the manuscript. Paul J. Gow contributed towards formulating the draft of the manuscript and critical revision. Peter W Angus contributed towards formulating the draft of the manuscript and critical revision. Mathis Grossmann contributed towards formulating the draft of the manuscript and critical revision.
Acknowledgements
Declaration of personal interests: There are no conflicts of interest to report in the writing of this manuscript. An Australian Postgraduate Award from the University of Melbourne to Marie Sinclair assisted in the production of this work.
Declaration of funding interests: None.




