Randomised clinical trial: safety, pharmacokinetics and pharmacodynamics of trazpiroben (TAK-906), a dopamine D2/D3 receptor antagonist, in patients with gastroparesis
The Handling Editor for this article was Professor Alexander Ford, and it was accepted for publication after full peer-review.
Summary
Background
Gastroparesis is a chronic gastric motility disorder. Dopamine D2/D3 receptor antagonists metoclopramide and domperidone are current treatment options but are associated with central nervous system and cardiovascular safety concerns, respectively, precluding chronic use. Trazpiroben (TAK-906), a dopamine D2/D3 receptor antagonist, is under development for chronic treatment of moderate-to-severe gastroparesis. Nonclinical data suggest trazpiroben will have D2/D3 receptor antagonism comparable with metoclopramide or domperidone.
Aims
To evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics (effect on prolactin and gastric function) of twice-daily trazpiroben (5, 25 and 100 mg) in participants with gastroparesis.
Methods
This phase 2a pilot study evaluated gastric emptying using the gastric emptying breath test, with metoclopramide as an internal control. Gastric accommodation and gastroparesis symptoms were assessed using the nutrient drink test and American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diary, respectively.
Results
Overall, 51 participants were enrolled. Trazpiroben was well tolerated, demonstrating a favourable safety profile without cardiovascular or central nervous system adverse events. All trazpiroben doses were rapidly absorbed and eliminated (t1/2z 4-5 hours), and D2/D3 receptor target engagement confirmed by increased serum prolactin (peaking at trazpiroben 25 mg). No effect on gastric emptying was demonstrated with trazpiroben or metoclopramide (P > 0.05), although benefits in volume-to-fullness were seen at trazpiroben 5 mg (P > 0.05) and 25 mg (88.5 vs −26.3 mL; P = 0.019), and nonsignificant numerical aggregate symptom score improvements were observed with trazpiroben 25 mg vs placebo (P = 0.182).
Conclusions
Trazpiroben was well tolerated with a favourable safety profile, supporting its further development for the treatment of gastroparesis. ClinicalTrials.gov identifier: NCT03268941.
1 INTRODUCTION
Gastroparesis is a chronic motility disorder of the stomach characterised by delayed gastric emptying in the absence of mechanical obstruction, such as pyloric stenosis.1, 2 Common symptoms reported by patients with gastroparesis include nausea, vomiting, belching and bloating, early satiety, abdominal pain and postprandial fullness. Symptoms are usually chronic and frequently fluctuate in severity.1, 3 The underlying mechanisms that predominantly lead to gastroparesis include neuromuscular dysfunction, involving derangement of extrinsic neural control (particularly vagal function), and dysfunction of the intrinsic nerves and interstitial cells involved in the local control of gastrointestinal muscle function.1 Gastroparesis may be idiopathic, or associated with diabetes mellitus or neurological disorders, or occur following a viral or bacterial infection, as well as following medical intervention (iatrogenic or postsurgical).1
Despite the overall low prevalence of gastroparesis, it has a disease burden similar to that of other important gastrointestinal diseases such as inflammatory bowel disease. The absence of approved drugs for the chronic treatment of gastroparesis together with its disease burden add to a large unmet medical need, particularly for patients with type 1 diabetes melitus.2, 4-8
Treatment options for patients with gastroparesis are limited. Dietary modifications, such as reduction in high-fat solid meals and replacement with small, frequent, liquid-based meals, or diets consisting of small particles that are digested easily interspersed with snacks to maintain caloric intake, are the first-line treatment options in all patients with gastroparesis regardless of severity.1, 9 If dietary modification proves unsuccessful, prokinetic and antiemetic medications such as metoclopramide (5-HT4 agonist, 5-HT3 and dopamine D2 antagonist) and domperidone (a dopamine D2/D3 receptor antagonist) can be prescribed.1, 10-15 Dopamine D2 and D3 receptors are validated targets in the treatment of gastroparesis, and antagonism of these receptors with metoclopramide and domperidone is effective in reducing symptoms in patients with gastroparesis, albeit with variable levels of effectiveness. However, both drugs are associated with safety concerns and unwanted adverse events (AEs), underlining the need for an efficacious treatment for gastroparesis with a favourable safety profile.2, 16-33
Trazpiroben (previously referred to as TAK-906 or ATC-1906M) is a D2/D3 receptor antagonist currently under development for the chronic treatment of patients with moderate-to-severe diabetic gastroparesis or idiopathic gastroparesis. Trazpiroben-induced D2/D3 receptor antagonism has been demonstrated in in vitro studies evaluating receptor binding affinity and activity, as well as in in vivo studies of prolactin secretion in rats and apomorphine-induced emesis in dogs.34
Based on preclinical pharmacology data, trazpiroben is anticipated to have comparable D2/D3 receptor antagonism to metoclopramide or domperidone but without their central nervous system or cardiovascular safety concerns. The zwitterionic structure of trazpiroben limits brain penetration, thereby decreasing central nervous system effects, whereas the low affinity of trazpiroben for the human ether-á-go-go-related gene potassium channel (IC50 of 15.6 µmol/L for trazpiroben vs 57 nmol/L for domperidone) reduces the potential for cardiac QTc prolongation/arrhythmia.34-38
Therefore, trazpiroben may represent an effective treatment for gastroparesis with a potentially favourable safety profile compared with currently available treatments in the same pharmacological class.
Our aim was to conduct a phase 2a pilot study to evaluate the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of trazpiroben in participants with gastroparesis.
2 MATERIALS AND METHODS
2.1 Study design
This study was a phase 2a, two-part, randomised, double-blind and open-label, placebo- or active-comparator-controlled trial (protocol registered and accessible at ClinicalTrials.gov [identifier: NCT03268941]) conducted at multiple US clinical research sites to evaluate the safety, tolerability, PK and PD of oral trazpiroben in participants with diabetic or idiopathic gastroparesis. In total, participants were screened at 8 study sites and enrolled at 7 sites. This study was conducted in compliance with Institutional Review Board (IRB) regulations, and in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice recommendations. The clinical study protocol, the investigators brochure, a sample informed consent form, and other study related documents were reviewed and approved by the local or central IRBs of all study sites. All participants agreed to participate by providing their written informed consent.
The study comprised two parts, preceded by a 7-day observation period to establish baseline gastric emptying breath test values, symptoms, satiety and study eligibility prior to enrolment.
Part 1 was a randomised, double-blind, placebo-controlled trial. A total of 48 participants (12 per treatment group) were planned for enrolment and allocated to one of four dosing treatment groups: trazpiroben 5 mg (group A), 25 mg (group B) and 100 mg (group C), and placebo (group D). Patients were enrolled by investigators at each clinical research site. The randomisation schedule was generated by Takeda Development Center Americas, Inc Analytical Sciences Department and stratified by underlying patient condition, with participants receiving unique randomisation numbers that assigned them to one of the three active treatment arms or the placebo arm of the trial to receive trazpiroben in a double-dummy manner. Blinding to study drug was maintained through the randomisation schedule, which was accessible to authorised personnel only; all participants, investigators and internal study personnel were blinded to treatment. Participants received study drug or placebo (in capsule form, all identical in appearance) for 9 consecutive days, resulting in a total of 17 doses (days 1-8 twice daily, day 9 once daily in the morning). The duration of 9 days was selected based on the PK profile of trazpiroben and deemed sufficient to achieve steady state levels of the drug and to observe an effect on efficacy.35
The first dose of study drug was administered after a fast of ≥8 hours; a test meal was given 1 hour after the first dose and, following this, participants remained fasted until 5 hours post-dose. At this time, participants received either a standardised clinical research unit lunch (days 1 and 7) or lunch ad libitum (days 2-6). The second dose was administered at least 1 hour post-lunch and before evening meal (Figure 1).
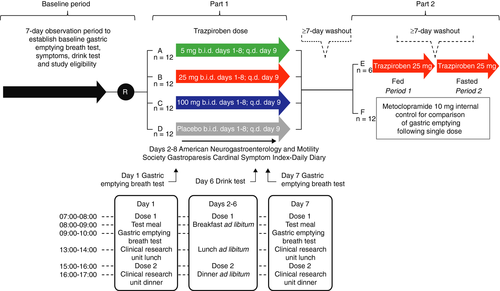
Part 2 was an open-label, active-comparator-controlled trial, consisting of two groups. In the first group (group E), the effect of food on the PK of trazpiroben was evaluated. In the second group (group F), metoclopramide 10 mg, a known motility agent, was used as an internal control for the evaluation of gastric emptying.15, 16, 39-41 Part 2 was open to all participants who completed part 1, following a washout of ≥7 days from the last dose in part 1. At the discretion of the investigator, participants from part 1 were enrolled into group E (six were planned) and allocated to receive a single dose of trazpiroben 25 mg, first with food (period 1) and then without food (period 2), in a crossover design separated by a washout period of ≥7 days. Additional participants from part 1 were enrolled into group F (12 were planned) and allocated to receive a single dose of metoclopramide 10 mg (Figure 1).
A minimum of four participants with diabetic gastroparesis and four participants with idiopathic gastroparesis were planned for enrolment into groups A-D of part 1 and group F of part 2. There was no patient or public involvement in the design, conduct or reporting of this study.
2.2 Inclusion and exclusion criteria
Men and women aged 18-75 years with diabetic or idiopathic gastroparesis who had a diagnosis of documented slow gastric emptying (delayed gastric emptying as determined by the gastric emptying breath test, defined as gastric half-emptying time [t1/2] ≥79 minutes [≥80th percentile of normative data]) at screening and a ≥3-month history of symptoms consistent with gastroparesis, including chronic postprandial fullness, abdominal pain, postprandial nausea, vomiting, loss of appetite and/or early satiety, were included.
Individuals with a history of clinically significant endocrine (apart from diabetes mellitus), gastrointestinal (intestinal obstruction), cardiovascular, haematological, hepatic, immunological, renal, respiratory, genitourinary or major neurological (including stroke and chronic seizures) abnormalities or diseases were excluded. Minor amendments were made to broaden the inclusion and exclusion criteria during the trial, namely increasing the upper age limit to 75 years and the maximum BMI to 40 kg/m2, to aid enrolment.
Full inclusion and exclusion criteria are presented in Table S1.
2.3 Trial objectives
The primary objective of this trial was to describe the safety and tolerability of trazpiroben treatment in participants with idiopathic or diabetic gastroparesis in the targeted population for the medication. Secondary objectives included evaluation of plasma PK parameters for trazpiroben, and assessments of the effects of trazpiroben on serum prolactin and on gastric t1/2 as measured by the gastric emptying breath test.
Exploratory objectives included evaluation of the impact of food intake on the PK of trazpiroben, changes in volume to fullness as determined by the nutrient drink test, the effect of trazpiroben on gastric emptying, and the effect of trazpiroben on symptoms of gastroparesis using the American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diary.
2.4 Schedule of study procedures
Details relating to the frequency of AE monitoring, PK/PD sampling, serum prolactin assessments, the gastric emptying breath test, nutrient drink test and American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diary are presented in Table S2.
2.4.1 Safety assessments
Safety was assessed continuously throughout the study. An AE was defined as any untoward medical occurrence in a study participant, namely, any unfavourable and unintended sign (eg, a clinically significant abnormal laboratory finding), symptom or disease temporally associated with use of a drug, whether or not it was considered related to the drug. AEs did not necessarily have to have a causal relationship with the study drug. A serious AE (SAE) was defined as any untoward medical occurrence that at any dose: (a) resulted in death; (b) was life threatening; (c) required inpatient hospitalisation or prolongation of existing hospitalisation; (d) resulted in persistent or significant disability and/or incapacity; (e) was a congenital anomaly/birth defect or (f) was an important medical event (further information provided in Text S1). AEs were graded as mild (usually transient and may require only minimal treatment or therapeutic intervention, does not generally interfere with usual activities of daily living), moderate (usually alleviated with additional specific therapeutic intervention, interferes with usual activities of daily living, causing discomfort but poses no significant or permanent risk of harm to the research participant) and severe (interrupts usual activities of daily living, or significantly affects clinical status, or may require intensive therapeutic intervention). AEs were recorded from the point of informed consent and at each study visit, but could be reported at any time during the study. AE collection continued for 14 days after the last dose of study drug, regardless of whether participants completed part 1 or part 2, or prematurely discontinued study drug treatment. All participants experiencing AEs were monitored until symptoms had reduced and any clinically relevant changes in laboratory values had returned to baseline, or a satisfactory explanation was provided.
The safety set comprised all participants who enrolled and received at least one dose of study drug; this set was used for demographic, baseline characteristics and safety summaries.
In part 1, safety data from the three trazpiroben treatment groups were summarised separately and pooled.
2.4.2 PK and PD evaluations
In part 1, the trazpiroben PK parameters derived were area under the concentration–time curve (AUC) during a dosing interval (AUCτ); maximum concentration (Cmax); time to first occurrence of Cmax (tmax; day 1 and day 7); terminal disposition phase half-life (t1/2z); and observed concentration at the end of a dosing interval (Ctrough; day 7).
In part 2, for group E (who received trazpiroben 25 mg, with food [period 1] and then without food [period 2] in a crossover design), AUC from time 0 to infinity (AUCinfinity; calculated using the observed value of the last quantifiable concentration), Cmax, tmax and t1/2z were derived. Similar PK outcomes were derived for metoclopramide but are not presented here.
The change in serum prolactin (a PD target engagement marker) from baseline to day 1 at tmax was measured by using a sandwich immunoassay (ADVIA Centaur Prolactin assay).
The PK analysis set comprised all enrolled participants who received at least one dose of study drug and had at least one measurable plasma concentration of trazpiroben (groups A-E) or metoclopramide (group F only). The PD analysis set was as described for the PK set and included participants with a baseline value and at least one valid post-baseline value for assessment of at least one of the PD measurements.
2.4.3 Gastric emptying breath test
Participants discontinued all gastroparesis-related medications at least 10 days prior to assessment of gastroparesis at baseline. The change from baseline to day 1 and day 7 following multiple doses of trazpiroben in part 1 and from baseline to day 1 for a single dose of metoclopramide in part 2 in gastric t1/2 was measured using the 13C-Spirulina platensis gastric emptying breath test.42
Prior to each gastric emptying breath test, participants underwent a fast of at least 8 hours before receiving study medication according to their study group (Figure 1). Two breath samples were collected prior to consumption of the test meal, which consisted of 100 mg of 13C-S. platensis, 27 g of freeze-dried egg mix, six saltine crackers and 180 mL of water. The caloric content of the meal was 238 kcal, and the meal had a balanced composition of 16.9 g of carbohydrate, 14.4 g of protein and 11.2 g of fat. The nature and size of the meal were selected to ensure stability at room temperature, palatability, caloric content and that it would be consumed entirely, even by patients with suspected gastroparesis and upper abdominal symptoms.
Following the test meal (1 hour post-dose), breath samples were collected at 15-minute intervals until 60 minutes post-meal, and then at 30-minute intervals until 4 hours post-meal (5 hours post-dose).
The gastric emptying breath test estimate of the corresponding gastric t1/2 value was computed using a simple linear interpolation algorithm. Analysis of gastric emptying breath test parameters was based on the full analysis set, excluding participants who were noncompliant with the test meal or test procedure (defined as consuming <90% of the test meal or having any incomplete breath sample).
2.4.4 Nutrient drink test
In part 1, meal sensitivity was assessed using the nutrient drink test (Ensure, Abbott Laboratories), a simple noninvasive test to appraise tolerance of ingested food.43 This was performed after a fast of at least 8 hours on day –2 and day 6. In previous studies, results from a combined barostat assessment of gastric accommodation and nutrient drink test of ingested food tolerance demonstrated correlation, especially in patients with upper gastrointestinal symptoms (dyspepsia).44
Gastroparesis symptoms were assessed via ingestion of 120 mL (4 oz) every 5 minutes of Ensure nutrient drink (1 kcal/mL; 11% fat, 73% carbohydrate, 16% protein) after a fast of at least 8 hours. Participants recorded their sensations at 5-minute intervals on a scale of 0-5 (0 = no symptoms, 3 = fullness sensation after a typical meal, 5 = maximum tolerated volume) to gain an assessment of volume to fullness and maximum tolerated volume. Nutrient intake was stopped when participants reached a score of 5, and the volume ingested was recorded. Postprandial symptoms of fullness, nausea, bloating and pain were measured 30 minutes after maximum tolerated volume using a 100-mm horizontal visual analogue scale, with no symptoms (labelled as “none”) to “worst ever” symptoms.
2.4.5 American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diary symptom scoring
The effect of trazpiroben on gastroparesis symptoms was assessed using the American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diary validated measure of gastroparesis symptoms, a patient-reported outcome instrument, which assesses nausea, early satiety, postprandial fullness and upper abdominal pain on a severity score from none (0) to very severe (4), in addition to the number of vomiting episodes during the previous 24 hours. A composite score is generated from the first four symptoms (nausea, early satiety, postprandial fullness and upper abdominal pain), with the core symptom score including all five symptoms (the first four symptoms plus vomiting frequency).45 American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diaries were completed daily, beginning 7 days prior to day 1 and continuing throughout the trial until the end of trial visit. The study was, however, not powered to demonstrate a symptomatic difference between groups using the American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diary.
2.4.6 Laboratory procedures and assessments
Evaluations of clinical/laboratory procedures and assessments, vital sign parameters and 12-lead electrocardiogram (ECG) results were also performed during the study. 46
2.5 Statistical considerations
2.5.1 Endpoints and data analysis
Primary, secondary and exploratory endpoints were analysed in participants randomised in part 1, and participants who received at least one dose of study drug in part 2. Plasma PK parameters for trazpiroben were summarised using descriptive statistics.
The primary endpoint was to provide a descriptive assessment of the safety and tolerability of trazpiroben in enrolled participants. Given the proposed sample size was small, this study was not sufficiently powered to detect unequivocal evidence of safety. The study sample size calculations were based on demonstrating efficacy for the secondary endpoint of gastric emptying, and assumed a standard deviation of 20% for the percentage change from baseline in gastric t1/2 as measured by the gastric emptying breath test (assuming baseline gastric half-emptying time equals 79 minutes based on the study inclusion criteria).42 It was estimated the study required 48 participants (12 per treatment group) to provide 80% power to detect a 25% difference in gastric emptying breath test between the trazpiroben dose groups using a two-sample, two-sided t-test with a significance level of 0.05.
2.5.2 Statistical analysis
An analysis of covariance model was used to analyse the change from baseline in prolactin, gastric t1/2 as measured by the gastric emptying breath test (part 1 at day 1 and day 7), volume to fullness measured using the nutrient drink test, and American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diary individual and composite scores. The model included fixed effects of stratification factor (diabetic/idiopathic gastroparesis) and regimen (dose level), and a covariate of baseline value; pairwise comparisons against placebo were performed within this model, with point estimates and 95% confidence intervals derived.
No multiplicity adjustment was performed for this exploratory study. All P values reported were nominal.
2.6 Data availability statement
Data pertaining to this study and the associated study protocol may be found at ClinicalTrials.gov (identifier: NCT03268941). Reuse of these data is not permitted. Additional data that support the findings of this study are available from Takeda Pharmaceuticals International upon reasonable request.
3 RESULTS
3.1 Demographic and baseline characteristics
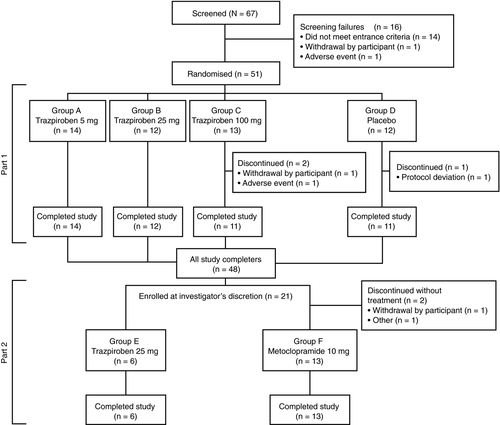
A total of 51 participants (34 [66.7%] with diabetic gastroparesis; 17 [33.3%] with idiopathic gastroparesis) were enrolled and randomised to each treatment group in part 1 of the study. The participant flow through parts 1 and 2 of the study is shown in Figure 2. The overall trial began when the first participant signed the trial informed consent form (September 2017), and ended when the last participant completed the last planned or follow-up visit/interaction associated with a planned visit (March 2018).
The mean age of all participants in part 1 was 53.4 years, and most participants were female (n = 40 [78.4%]). Overall, enrolled participants had mild-to-moderate disease based on gastric emptying rates at baseline, and in accordance with previous studies (mild 11%-20% gastric retention; moderate >20% to 35% gastric retention).42, 47, 48
Of the 51 participants who entered the study, 21 enrolled in part 2 and were allocated (at the discretion of the investigator) to receive a single dose of trazpiroben 25 mg (n = 6) or metoclopramide 10 mg (n = 15). The mean age of participants in part 2 was comparable to that in part 1 (trazpiroben 25 mg, 58.3 years; metoclopramide 10 mg, 53.6 years vs 53.4 years). The proportion of females in the trazpiroben 25 mg arm of part 2 was lower than that of part 1 (33.3% vs 83.3%), and all participants in the metoclopramide 10 mg arm of part 2 were female.
Demographic and baseline characteristics of participants in part 1 and part 2 are shown in Table 1.
| (A) Part 1 | |||||
|---|---|---|---|---|---|
| Group A | Group B | Group C | Group D |
Total (N = 51) |
|
|
Trazpiroben 5 mg (N = 14) |
Trazpiroben 25 mg (N = 12) |
Trazpiroben 100 mg (N = 13) |
Placebo (N = 12) |
||
| Age (years) | |||||
| Mean (SD) | 54.7 (11.8) | 52.7 (17.1) | 55.9 (10.2) | 49.9 (13.8) | 53.4 (13.1) |
| Sex (n [%]) | |||||
| Male | 3 (21.4) | 2 (16.7) | 3 (23.1) | 3 (25.0) | 11 (21.6) |
| Female | 11 (78.6) | 10 (83.3) | 10 (76.9) | 9 (75.0) | 40 (78.4) |
| Ethnicity (n [%]) | |||||
| Hispanic or Latino | 5 (35.7) | 3 (25.0) | 7 (53.8) | 9 (75.0) | 24 (47.1) |
| Non-Hispanic and Latino | 9 (64.3) | 9 (75.0) | 6 (46.2) | 3 (25.0) | 27 (52.9) |
| Race (n [%]) | |||||
| Black or African American | 1 (7.1) | 4 (33.3) | 2 (15.4) | 1 (8.3) | 8 (15.7) |
| Whitea | 13 (92.9) | 7 (58.3) | 11 (84.6) | 11 (91.7) | 42 (82.4) |
| European | 4 (30.8) | 5 (71.4) | 3 (27.3) | 5 (45.5) | 17 (40.5) |
| Not reported | 9 (69.2) | 2 (28.6) | 8 (72.7) | 6 (54.5) | 25 (59.5) |
| Multiracial | 0 | 1 (8.3) | 0 | 0 | 1 (2.0) |
| Body mass index (kg/m2) | |||||
| Mean (SD) | 29.7 (4.5) | 29.8 (4.5) | 32.6 (4.4) | 27.3 (4.2) | 29.9 (4.7) |
| Min, max | 22.3, 38.8 | 22.8, 37.1 | 25.7, 39.9 | 21.2, 34.0 | 21.2, 39.9 |
| Underlying disease (n [%]) | |||||
| Diabetic gastroparesis | 10 (71.4) | 8 (66.7) | 8 (61.5) | 8 (66.7) | 34 (66.7) |
| Idiopathic gastroparesis | 4 (28.6) | 4 (33.3) | 5 (38.5) | 4 (33.3) | 17 (33.3) |
| Nutrient drink test (day –2) | |||||
| Mean (mL [SD]) | 381.8 (204.2) | 371.8 (190.4) | 366.6 (98.3)a | 388.2 (166.9) | — |
| Min, max | 177, 999 | 118, 710 | 237, 474 | 118, 711 | — |
| Gastric emptying (gastric emptying breath test baseline) | |||||
| Mean (SD) | 130.2 (35.3) | 121.3 (33.6)a | 122.5 (31.8) | 118.7 (43.1)a | — |
| Min, max | 88.7, 214.0 | 79.9, 184.3 | 70.5, 183.4 | 72.5, 224.2 | — |
| American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diary composite score | |||||
| Mean (SD) | 2.4 (0.6) | 2.2 (0.5) | 2.4 (0.4) | 2.5 (0.6) | — |
| Min, max | 0.9, 3.3 | 1.4, 2.9 | 1.7, 2.9 | 1.7, 3.6 | — |
| (B) Part 2 | ||
|---|---|---|
| Group E | Group F | |
|
Trazpiroben 25 mg (N = 6) |
Metoclopramide 10 mg (N = 13) |
|
| Age (years) | ||
| Mean (SD) | 58.3 (6.5) | 53.6 (10.8) |
| Sex (n [%]) | ||
| Male | 4 (66.7) | 0 |
| Female | 2 (33.3) | 13 (100) |
| Ethnicity (n [%]) | ||
| Hispanic or Latino | 3 (50.0) | 7 (53.8) |
| Non-Hispanic and Latino | 3 (50.0) | 6 (46.2) |
| Race (n [%]) | ||
| Black or African American | 0 | 2 (15.4) |
| Whiteb | 6 (100) | 11 (84.6) |
| European | 1 (16.7) | 4 (36.4) |
| Not reported | 5 (83.3) | 7 (63.6) |
| Body mass index (kg/m2) | ||
| Mean (SD) | 28.7 (2.0) | 30.7 (4.9) |
| Min, max | 26.0, 31.7 | 21.2, 38.0 |
| Underlying disease (n [%]) | ||
| Diabetic gastroparesis | 4 (66.7) | 9 (69.2) |
| Idiopathic gastroparesis | 2 (33.3) | 4 (30.8) |
- Abbreviation: SD, standard deviation.
- a n = 11.
- b Percentages of subcategories are based on the number of participants who are White.
3.2 Participant disposition
In part 1, the study was completed by 48 participants (94.1%). Three patients prematurely discontinued the study drug, one from the placebo group (protocol deviation) and two from the trazpiroben 100 mg group (AE, n = 1; withdrawal by participant, n = 1). Full patient disposition details for part 1 are presented in Table S3.
In part 2, all six participants allocated to receive trazpiroben 25 mg completed the study. In the metoclopramide 10 mg group (group F), of the 15 participants enrolled, 13 received the study drug and completed the study. Two participants discontinued prior to receiving the study drug owing to ‘participant withdrawal’ and ‘other reason’ (closure of the diabetic arm of the study).
3.3 Safety
An overview of the treatment-emergent AEs (TEAEs) and SAEs reported in both parts of the study is presented in Table 2 and Table S4. There were no deaths in the study and only one severe TEAE was observed, which occurred in part 1 in a participant in group D (placebo). The majority of TEAEs were mild or moderate in severity. Only one participant (group C, trazpiroben 100 mg) discontinued the study drug owing to an SAE of urinary tract infection and acute kidney injury, both of which were considered moderate in severity and not related to the study drug.
| (A) Part 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group A | Group B | Group C | Group D | |||||
|
Trazpiroben 5 mg (N = 14) |
Trazpiroben 25 mg (N = 12) |
Trazpiroben 100 mg (N = 13) |
Placebo (N = 12) |
|||||
|
Events (n) |
Participants (n [%]) |
Events (n) |
Participants (n [%]) |
Events (n) |
Participants (n [%]) |
Events (n) |
Participants (n [%]) |
|
| Overview of TEAEs and SAEs | ||||||||
| AEs | 4 | 4 (28.6) | 4 | 3 (25.0) | 9 | 4 (30.8) | 7 | 4 (33.3) |
| Relateda | 2 | 2 (14.3) | 2 | 2 (16.7) | 1 | 1 (7.7) | 4 | 1 (8.3) |
| Not related | 2 | 2 (14.3) | 2 | 1 (8.3) | 8 | 3 (23.1) | 3 | 3 (25.0) |
| Mild | 1 | 1 (7.1) | 3 | 2 (16.7) | 6 | 3 (23.1) | 6 | 3 (25.0) |
| Moderate | 3 | 3 (21.4) | 1 | 1 (8.3) | 3 | 1 (7.7) | 0 | |
| Severe | 0 | 0 | 0 | 1 | 1 (8.3) | |||
| Leading to discontinuation | 0 | 0 | 1 | 1 (7.7) | 0 | |||
| SAEs | 0 | 0 | 2 | 1 (7.7) | 1 | 1 (8.3) | ||
| Relateda | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Not related | 0 | 0 | 2 | 1 (7.7) | 1 | 1 (8.3) | ||
| Leading to discontinuation | 0 | 0 | 0 | 1 (7.7) | 0 | |||
| Deaths | 0 | 0 | 0 | 0 | ||||
| (B) Part 2 | ||||||
|---|---|---|---|---|---|---|
| Group E | Group F | |||||
| Trazpiroben 25 mg | Metoclopramide 10 mg | |||||
|
Fed (N = 6) |
Fasted (N = 6) |
(N = 13) | ||||
|
Events (n) |
Participants (n [%]) |
Events (n) |
Participants (n [%]) |
Events (n) |
Participants (n [%]) |
|
| Overview of TEAEs and SAEs | ||||||
| AEs | 1 | 1 (16.7) | 0 | 1 | 1 (7.7) | |
| Relateda | 0 | 0 | 1 | 1 (7.7) | ||
| Not related | 1 | 1 (16.7) | 0 | 0 | ||
| Mild | 0 | 0 | 0 | |||
| Moderate | 1 | 1 (16.7) | 0 | 1 | 1 (7.7) | |
| Severe | 0 | 0 | 0 | |||
| Leading to discontinuation | 0 | 0 | 0 | |||
| SAEs | 0 | 0 | 0 | |||
| Relateda | 0 | 0 | 0 | |||
| Not related | 0 | 0 | 0 | |||
| Leading to discontinuation | 0 | 0 | 0 | |||
| Deaths | 0 | 0 | 0 | |||
- Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ECG, electrocardiogram; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
- a ‘Related’ was defined as an AE that followed a reasonable temporal sequence from administration of a drug (including the course after withdrawal of the drug), or for which a causal relationship was at least a reasonable possibility; that is, the relationship could not be ruled out, although factors other than the drug, such as underlying diseases, complications, concomitant drugs and concurrent treatments, may also have been responsible.
In part 1, the proportions of participants experiencing AEs were similar across all treatment groups (25.0%-33.3%), and there were no meaningful differences or trends in the proportions of participants experiencing drug-related TEAEs. No AEs were reported in more than one participant in any trazpiroben dose group except for fatigue, which was reported in two of 14 participants (14.3%) receiving trazpiroben 5 mg.
In part 2, one participant in group E (trazpiroben 25 mg) reported a TEAE (urinary tract infection) that was moderate in severity and considered unrelated to the study drug. One participant in group F (metoclopramide 10 mg) reported a TEAE (muscle spasms) that was of moderate severity and considered related to metoclopramide. There were no AEs or SAEs leading to discontinuations.
Across part 1 and part 2, there were no significant safety issues or concerns attributable to trazpiroben, including any clinically meaningful ECG changes or extra-pyramidal symptoms.
3.4 PK and PD measurements
Trazpiroben was rapidly absorbed and eliminated across all treatment groups, with a t1/2z elimination time of ~4-5 hours (Figure 3). Cmax and AUC values increased with increasing dose, and minimal accumulation of trazpiroben was observed with twice-daily administration (ratio of day 7 vs day 1 AUCτ ranged from 1.26 to 1.34 across dose groups).
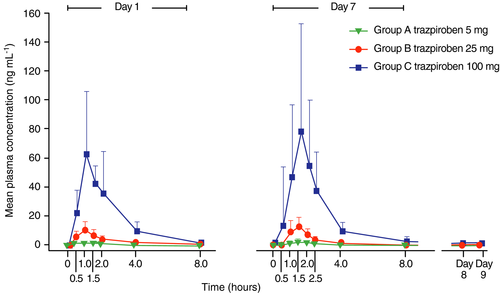
Serum prolactin levels increased in response to treatment with trazpiroben on day 1 and day 7, with maximal pharmacology achieved at a dose of trazpiroben 25 mg (based on ratios of day 1 Cmax to baseline concentrations; Figure S1); demonstrated by no additional increase in serum prolactin concentration with trazpiroben 100 mg. For most participants, serum prolactin concentrations returned to normal (baseline) levels 8 hours post-trazpiroben dose. Similar serum prolactin concentration changes were observed in participants with diabetic gastroparesis and those with idiopathic gastroparesis.46
In part 2, no difference in total exposure (AUC to the last measurable concentration [AUClast] and AUCinfinity) of trazpiroben was observed between fed (period 1) and fasted (period 2) participants (Figure S2). In fed participants, the geometric mean Cmax of trazpiroben 25 mg was reduced by approximately 33% compared with fasted participants.
3.4.1 Gastric emptying
In part 1, no statistically significant effect on change in gastric emptying time from baseline was observed with trazpiroben compared with placebo from baseline to day 1 or baseline to day 7. Similar observations were noted in part 2 for the internal active control metoclopramide 10 mg (Figure S3).
3.4.2 Nutrient drink test
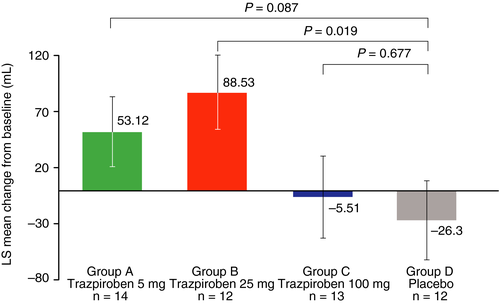
After 1 week of treatment with trazpiroben, increases in mean volume to fullness were observed in participants receiving trazpiroben 5 mg or 25 mg compared with placebo. These increases in volume to fullness were significant for trazpiroben 25 mg compared with placebo (88.5 mL vs −26.3 mL; P = 0.019). No effect on volume to fullness was observed for trazpiroben 100 mg (Figure 4).
3.4.3 American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diary symptom scoring
Overall, a trend towards improvement (reduction) in American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diary symptom scores was observed in all treatment groups following 9 consecutive days of treatment, but no significant treatment benefits vs placebo were demonstrated. Reductions in symptom scores were numerically greater in participants receiving trazpiroben compared with those receiving placebo, with the greatest numerical differences observed for nausea, postprandial fullness and patient-rated overall severity of gastroparesis symptoms (Figure S4). The overall reductions in symptom scores from baseline were similar across all groups when a composite endpoint of nausea, early satiety, postprandial fullness and upper abdominal pain symptom scores was used (Table S5). Similar observations were made in participants with diabetic gastroparesis and those with idiopathic gastroparesis (data not shown).46
4 DISCUSSION
This study demonstrated that twice-daily dosing of trazpiroben over 9 consecutive days was not associated with any clinically significant safety or tolerability issues, and had a favourable safety profile, based on the overall observed AE profile, vital signs, clinical laboratory parameters and ECGs, in participants with gastroparesis and when stratified by underlying disease condition (diabetic or idiopathic gastroparesis).
Trazpiroben appeared to be well tolerated, with the majority of TEAEs reported as mild to moderate in severity. Only two participants (one from the placebo group and one from the trazpiroben 100 mg group) reported SAEs, neither of which was related to trazpiroben. Alternative D2/D3 receptor antagonists used for the treatment of gastroparesis, such as metoclopramide and domperidone, have been associated with extra-pyramidal symptoms including rare occurrences of tardive dyskinesia and cardiovascular events (QT prolongation), respectively.11, 14, 49 In this study, no extra-pyramidal symptoms or cardiovascular events of concern were reported in participants receiving trazpiroben, although one participant experienced palpitations (trazpiroben 25 mg) and one experienced tremor (trazpiroben 100 mg), which were medically reviewed in the context of current clinical and nonclinical information. In the participant who experienced palpitations, no ECG abnormalities or any other cardiovascular events were noted, and all other vital signs were within normal limits. In the participant who experienced tremor, this event occurred in the absence of other signs or symptoms suggestive of neurological origin, and the event resolved within 1 day. Both participants completed the study. However, it must be acknowledged that this study was of 9 days duration only, and further investigation of the safety profile of trazpiroben over longer time periods and in a much larger patient population is required.
Trazpiroben demonstrated rapid absorption and elimination across all doses studied, with minimal accumulation and approximately proportional increases in Cmax and AUC with increasing doses. Food did not appear to exert an effect on overall drug exposure, based on AUC measurements, although a reduction in Cmax (33%) was noted in participants receiving trazpiroben 25 mg after food.
The increases in serum prolactin confirmed D2/D3 receptor target engagement, and suggested that PD activity was associated with receptor target engagement. The highest levels of serum prolactin were achieved with trazpiroben 25 mg, suggesting that at the resulting concentration maximal receptor antagonism may have been achieved. These data were consistent with in vitro studies of receptor binding affinity and activity and in vivo studies of effects on prolactin secretion in rats and apomorphine-induced emesis in dogs, as well as the first-in-human study of trazpiroben; AT-01C (NCT03544229).50
Trazpiroben was unable to produce any significant effect on gastric emptying measured by the gastric emptying breath test in part 1; however, no effect on gastric emptying was demonstrated with the internal active control metoclopramide in part 2 either. With respect to an absence of effect with metoclopramide, this may be related to the dosing regimen (10 mg administered once daily) and future studies may benefit from additional treatment arms with increased doses (up to 20 mg) and frequency (four times daily).39, 40 It is noteworthy that domperidone, a promotility agent and D2 receptor antagonist, has also demonstrated inconsistent results with respect to its ability to accelerate gastric emptying, with a recent meta-analysis noting that, based on available studies, domperidone did not exert a significant effect on gastric t1/2.31, 47, 51 The absence of an effect on gastric emptying in this study is therefore not entirely unexpected, and alternative measures of gastric emptying, such as scintigraphy, may be required to confirm the observations of the gastric emptying breath test, as previous studies have demonstrated there is good correlation between scintigraphy and breath test methods of assessing gastric emptying.1, 52-54
Participant symptom scores (particularly for postprandial fullness and nausea, and patient-rated overall severity of gastroparesis symptoms) were numerically greater (with the exception of upper abdominal pain) in all trazpiroben treatment groups compared with placebo, albeit without statistical significance. Symptoms of postprandial fullness and nausea, vomiting and early satiety, but not abdominal pain and bloating, have been reported as being associated with delayed gastric emptying, and is broadly in line with the trend observed in this study.2, 55, 56 Although the study was not powered to demonstrate a symptomatic difference between groups, and the study was conducted over only 9 days, a trend towards improved American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diary symptom scores was observed, suggesting trazpiroben may be associated with symptom benefit. These data will therefore prove useful to inform sample size and power estimation calculations for future studies.
While gastric emptying is the hallmark for the diagnosis of gastroparesis, there may be various other mechanisms which produce and influence gastroparesis symptoms.52 A significant increase in volume to fullness with the trazpiroben 25 mg dose was observed, which may explain the trend in American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diary symptom scores; particularly patient-rated overall severity scores, for which the response appeared to correlate reciprocally with dose. The mechanism for the positive effect of the drug on volume to fullness may be related to the previously documented reduction in upper gastrointestinal symptoms with dopaminergic antagonists, which led to participants' ability to tolerate a greater volume of the nutrient drink.57 Dopamine modulation may therefore alter gastric accommodation, leading to an increase in volume tolerated.
However, it must be noted that volume to fullness is a subjective outcome measure developed for use in functional dyspepsia rather than gastroparesis, meaning the importance of these findings should not be overstated in relation to objective measures such as gastric emptying.58
The curve profiles for volume to fullness and American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diary symptom scores were consistent with the PK/PD data, indicating that trazpiroben 25 mg produces the greatest response and that higher doses do not result in continued improvement. This is consistent with observations of other gastrointestinal promotility agents, such as erythromycin, for which high doses (>500 mg) induce strong antral contractions, but are also associated with gastrointestinal AEs such as upper abdominal pain and nausea.59-61
There are several limitations to this study. First, the population was relatively homogeneous with respect to disease severity, with most participants presenting with mild-to-moderate disease; this may have impacted the ability to demonstrate a positive effect for outcomes such as gastric emptying. Second, because this was a pilot study, the number of participants was small, limiting the statistical power. Therefore, for endpoints such as the exploratory endpoint American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diary symptom scoring, there was insufficient power to demonstrate statistically significant symptomatic differences between treatment groups. Finally, the duration of trazpiroben treatment in this study was short at only 9 days. For treatments that are intended to be used in a chronic and/or continuous setting, longer treatment periods of at least 8 weeks would be preferable to evaluate changes in symptoms of gastroparesis and possibly the effect of trazpiroben on gastric emptying. This short treatment period (9 days) may also have impacted the outcomes of the American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diary symptom scoring tool, which recommends that symptoms are recorded over a 4- to 8-week period, because treatment for gastroparesis is associated with a progressive decline in American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diary scores in patients over the course of 8 weeks.45, 62, 63
In conclusion, the results from this short-term, phase 2a, pilot study demonstrate that trazpiroben is well tolerated with a favourable safety profile, without any evidence of cardiovascular or central nervous system effects. Despite the absence of a significant effect on gastric emptying, trazpiroben 25 mg produced significant benefits in volume to fullness and demonstrated numerical improvements in symptom scores. PK and PD analyses demonstrated that trazpiroben 25 mg was the dose that was expected to achieve maximal receptor antagonism. These data support the further development of trazpiroben 25 mg for the treatment of diabetic and idiopathic gastroparesis, and a larger phase 2b clinical study is currently recruiting to evaluate the efficacy and safety of trazpiroben in participants with symptomatic idiopathic or diabetic gastroparesis (NCT03544229).
ACKNOWLEDGEMENT
Steve Banner, PhD, of Oxford PharmaGenesis, Oxford, UK, provided medical writing assistance, which was supported by Takeda Pharmaceutical Company, Ltd.
Declaration of personal interests: BK and MC serve as advisors to Takeda Pharmaceuticals and conduct research supported by Takeda Pharmaceuticals. They received no personal financial remuneration. CS was a Takeda employee at the time the study was performed and was involved in the trial oversight, interpretation of the data and writing the clinical study report; she is currently a Medical Director at Rhythm Pharmaceuticals Inc, Boston, MA, USA. GD was a contracted employee of Takeda Pharmaceuticals InternationalCo. at the time the study was performed. WZ is an employee of Takeda Pharmaceuticals, the study sponsor that provided funding for this study. SG was a Takeda employee at the time the study was performed and was the biomarker lead for the programme. CC was a Takeda employee at the time the study was performed; he is currently at Bayer Pharmaceuticals, Boston, MA, USA. EC was a Takeda employee at the time the study was performed; he is currently Vice President at Precision Medicine, San Diego, CA, USA. The study sponsor was involved in the design of the study, interpretation of the data, and preparation, review and approval of the manuscript.
AUTHORSHIP
Guarantor of the article: Dr Braden Kuo.
Author contributions: CS, GD, WZ, SG, EC and CC were involved in the conception, organisation and execution of the study. WZ designed the statistical analysis. BK, CS, GD, WZ, SG, CC and MC were involved in all stages of the development of the manuscript and provided review and critical analysis. All authors had complete access to the data that support this manuscript, and all authors approved the final version of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




