Risk factors for neonatal brachial plexus palsy: a systematic review and meta-analysis
Abstract
enAim
To provide a comprehensive update on the most prevalent, significant risk factors for neonatal brachial plexus palsy (NBPP).
Method
Cochrane CENTRAL, MEDLINE, Web of Science, Embase, and ClinicalTrials.gov were searched for relevant publications up to March 2019. Studies assessing risk factors of NBPP in relation to typically developing comparison individuals were included. Meta-analysis was performed for the five most significant risk factors, on the basis of the PRISMA statement and MOOSE guidelines. Pooled odds ratios (ORs), 95% confidence intervals (CIs), and across-study heterogeneity (I2) were reported. Reporting bias and quality of evidence was rated. In addition, we assessed the incidence of NBPP.
Results
Twenty-two observational studies with a total sample size of 29 419 037 live births were selected. Significant risk factors included shoulder dystocia (OR 115.27; 95% CI 81.35–163.35; I2=92%), macrosomia (OR 9.75; 95% CI 8.29–11.46; I2=70%), (gestational) diabetes (OR 5.33; 95% CI 3.77–7.55; I2=59%), instrumental delivery (OR 3.8; 95% CI 2.77–5.23; I2=77%), and breech delivery (OR 2.49; 95% CI 1.67–3.7; I2=70%). Caesarean section appeared as a protective factor (OR 0.13; 95% CI 0.11–0.16; I2=41%). The pooled overall incidence of NBPP was 1.74 per 1000 live births. It has decreased in recent years.
Interpretation
The incidence of NBPP is decreasing. Shoulder dystocia, macrosomia, maternal diabetes, instrumental delivery, and breech delivery are risk factors for NBPP. Caesarean section appears as a protective factor.
What this paper adds
- The overall incidence of neonatal brachial plexus palsy is 1.74 per 1000 live births.
- The incidence has declined significantly.
- Shoulder dystocia, macrosomia, maternal diabetes, instrumental delivery, and breech delivery are the main risk factors.
- Prevention is difficult owing to unpredictability and often labour-related risk.
Resumen
esFactores de riesgo para parálisis braquial neonatal: una revisión sistemática y metaanálisis
Objetivo
Proporcionar una actualización completa en los factores de riesgo más prevalentes y significantes para parálisis braquial neonatal (PBN, parálisis de Erb-Duchenne).
Metodo
Se realizaron búsquedas en Cochrane CENTRAL, MEDLINE, páginas de ciencia, Embase, y ClinicalTrials.gov para obtener publicaciones relevantes hasta Marzo del 2019. Se incluyeron estudios que evalúan factores de riesgo para PBN en comparación con niños con desarrollo típico. Se realizó un metaanálisis de los primeros cinco factores de riesgo más significativos sobre la base de declaración PRISMA y las guías MOOSE. Se informaron los odds ratios (OR) agrupados, los intervalos de confianza (IC) del 95 % y la heterogeneidad entra estudios (I2). Se calificó el sesgo de informe y la calidad de la evidencia. Además, evaluamos la incidencia de PBN.
Resultados
Se seleccionaron 22 estudios observacionales con una muestra total de 29.419.037 nacidos vivos. Los factores de riesgo significantes incluyeron: distocia de hombros (OR 115.27; 95% CI 81.35-163.35; I2= 92%), macrosomía (OR 9.75; 95% CI 8.29-11.46; I2= 70%), diabetes gestacional (OR 5.33; 95% CI 3.77-7.55; I2= 59%), parto instrumental (OR 3.8; 95% CI 2.77-5.23 I2= 77%) y presentación podálica (OR 2.49; 95% CI 1.67-3.7; I2= 70%). La cesárea apareció como un factor protector (OR 0.13; 95% CI 0.11-0.16 I2= 41%). La incidencia global combinada de PBN fue de 1.74 por 1000 nacidos vivos. Ha disminuido en los últimos años.
Interpretacion
La incidencia de PBN está disminuyendo. La distocia de hombros, macrosomía, diabetes gestacional, parto instrumental, y presentación podálica son factores de riesgo para PBN. La cesárea aparece como factor protector.
Resumo
ptFatores de risco para paralisia neonatal do plexo braquial: uma revisão sistemática e metanálise
Objetivo
Fornecer uma atualização abrangente sobre os fatores de risco mais prevalentes e significantes para a paralisia neonatal do plexo braquial (PNPP, paralisia de Erb-Duchenne).
Método
Cochrane CENTRAL, MEDLINE, Web of Science, Embase, e ClinicalTrials.gov foram pesquisados quanto a publicações relevantes até março de 2019. Estudos avaliando os fatores de risco para PNPP em relação a indivíduos com desenvolvimento típico foram incluídos. Foi realizada metanálise para os cinco fatores de risco mais significativos, com base nas diretrizes PRISMA e MOOSE. As taxas de risco agrupadas (TRs), intervalos de confiança a 95% (ICs), e heteogeneidade entre estudos (I2) foram reportados. O viés de relato e qualidade da evidência foram pontuados. Adicionalmente, avaliamos a incidência de PNPP.
Resultados
Vinte e dois estudos observacionais com amostra total de 29.419.037 nascidos vivos foram selecionados. Fatores de risco significativos incluíram distocia de ombro, (TR 115,27; IC 95% 81,35–163,35; I2=92%), macrossomia (TR 9,75; IC 95% 8,29–11,46; I2=70%), diabetes (gestational) (TR 5,33; IC 95% 3,77–7,55; I2=59%), parto instrumental (TR 3,8; IC 95% 2,77–5,23; I2=77%), e apresentação pélvica (TR 2,49; IC 95% 1,67–3,7; I2=70%). Parto cesárea foi um fator protetor (TR 0,13; IC 95% 0,11–0,16; I2=41%). A incidência agrupada geral de PNPP foi 1,74 por 1.000 nascidos vivos. A quantidade diminuiu nos últimos anos.
Interpretação
A incidência de PNPP tem diminuído. Distocia de ombro, macrossomia, diabetes materna, parto instrumental e apresentação pélvica são fatores de risco para PNPP. Parto cesárea parece ser um fator protetor.
What this paper adds
en
- The overall incidence of neonatal brachial plexus palsy is 1.74 per 1000 live births.
- The incidence has declined significantly.
- Shoulder dystocia, macrosomia, maternal diabetes, instrumental delivery, and breech delivery are the main risk factors.
- Prevention is difficult owing to unpredictability and often labour-related risk.
This article's abstract has been translated into Spanish and Portuguese.
Follow the links from the abstract to view the translations.
This article is commented on by Gherman on page 662 of this issue.
Editor's Choice
Neonatal brachial plexus palsy results in a high, complex, long-lasting burden. There is a wide variety in epidemiological reports and the predicting value of classically considered risk factors has been questioned. My Editor’s Choice for the June 2020 issue is this comprehensive systematic review and meta-analysis of risk factors identified in the literature reporting collectively on nearly 30 million births.1 It clarifies a number of issues and also stresses challenges associated to this type of enterprise, including with regard to appraisal tools.2,3 To date, no intervention has effectively prevented the majority of cases of neonatal brachial plexus palsy.4 Hopefully the presented results will provide a firmer base for designing and studying intervention strategies in the future.
REFERENCES
1. Van der Looven R, Le Roy L, Tanghe E, et al. Risk factors for neonatal brachial plexus palsy: a systematic review and meta-analysis. Dev Med Child Neurol 2020; 62: 673–83.
2. Rehm A, Thahir A. Validity of a meta-analysis of risk factors for neonatal brachial plexus palsies. Dev Med Child Neurol 2020; 62: 763.
3. Van der Looven R, Le Roy L, Pauwels N, Vingerhoets G. Critical appraisal tools and rater training in systematic reviews and meta-analyses. Dev Med Child Neurol 2020 62: 764.
4. Gherman R. Are there specific interventions that may reduce the incidence of neonatal brachial plexus palsy? Dev Med Child Neurol 2020; 62: 662.
Video Podcast: https://youtu.be/Yd3gy7aMJXE
Abbreviation
-
- NBPP
-
- Neonatal brachial plexus palsy
Neonatal brachial plexus palsy (NBPP) is reported worldwide in 0.1 to 8.1 per 1000 live births.1-6 Incidence rates vary with study type and the availability of maternal and fetal care.7-9 NBPP is the result of a closed nerve stretch injury to the brachial plexus, mostly occurring during labour. The mechanisms of injury include maternal, obstetric, and infant factors that apply traction on the anatomically vulnerable plexus.
Early management includes parental counselling, family support, splinting, and appropriate and supervised rehabilitation. Neurosurgical intervention is usually undertaken at 3 to 6 months of age in children who have shown little or no significant improvement in the affected muscle groups.2, 10-15 Depending on the long-term clinical course, secondary surgery may be indicated later in life.
The few studies on NBPP prognosis not hampered by selection bias suggest that residual deficits are estimated at 20% to 35%.3, 5, 16-20 This finding is at odds with the previous optimistic view of full recovery in over 90% of affected children.21-26 After neonatal occurrence, the upper limb paresis affects psychomotor development, bone growth, and joint development. Deformities occur and may cause painful arthrosis in adults. Children with incomplete recovery have considerable risk for long-term functional limitations, with financial burden, restricted daily life activities, limited participation, and important overall quality of life implications.16, 27-43
The potential impact of NBPP on the child’s physical and psychological development, and their socio-economic future, highlights the need for the development of preventive strategies. Early identification of modifiable risk factors is therefore imperative. Appropriate management or avoidance of risk factors might contribute to a reduction in NBPP morbidity.
The aim of the present study was to determine and unravel the significant risk factors for NBPP to enable individualized risk assessment and proper counselling of individual women at risk. We conducted a meta-analysis by combining the results from all available studies to: (1) assess the five most frequently reported significant risk factors; (2) calculate their pooled odds ratios; and (3) assess the heterogeneity among studies. In addition, we assessed the incidence and its evolution.
Method
The Cochrane Database of Systematic Reviews was searched to ensure a similar review had not been undertaken. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement,44 MOOSE guidelines,45 and Grant’s review46 were followed to report this meta-analysis. The protocol was accepted for registration in PROSPERO on 14th November 2017 (CRD42017076642).
Information sources and search strategy
The search strategy, using the Population, Intervention, Comparison, and Outcome (PICO)47 format for clinical questions, was developed systematically in the MEDLINE database (PubMed interface), using medical subject headings as well as free text words (Fig. S1, online supporting information). We searched entries on ClinicalTrials.gov for ongoing studies. A final literature search was conducted on 1st March 2019. Manual reference screening of retrieved articles was performed to identify other pertinent studies. Until the time of submission of this paper for publication, an automatic e-mail alert was checked for possible additional relevant articles. A list of all identified studies is available on request. We imported all citations into bibliographic software (Endnote X7; Thomson Reuters, Philadelphia, PA, USA).48
Eligibility
We included all published randomized controlled trials and observational studies (cohort and case–control) that assessed the risk of NBPP as the primary outcome in relation to typically developing comparison individuals. NBPP was defined as a closed nerve stretch injury to the brachial plexus (C5–T1). To limit population bias, studies with only ‘upper’ plexus infants or with unclear definition such as ‘shoulder injury’ were excluded. Language was restricted to English, Dutch, or French. Studies with non-extractable data were excluded.
Three authors (RVDL, LLR, and ET) independently screened all articles by title, abstract, and keywords using the Covidence web-based platform49 recommended by the Cochrane Organization for systematic reviews. When all inclusion criteria (Table S1, online supporting information) were met, the full text version of the article was assessed. Disagreements were resolved by consensus.
Data extraction
Data from each included trial were extracted independently by the three reviewers (RVDL, LLR, ET) on a data extraction form designed in accordance with the Cochrane Checklist of items.50 Any disagreements were solved after discussion. Details included source, eligibility, study design, participants, risk factors, results, as well as any other miscellaneous data. The primary outcome was risk factors. Secondary, significant risk factors, considered as p<0.05, were extracted from the articles and listed. The five most frequently reported significant risk factors were subjected to meta-analysis. Raw data for case and control groups were included for further analysis. Percentages were converted into n-values with rounding up if ≥0.5. Macrosomia was defined as birthweight above 4000g.51 Birthweight was categorized as 4000 to 4499g, 4500 to 4999g, and 5000g or more.
Risk of bias and quality assessment
Each reviewer independently assessed the risk of bias by using the Newcastle-Ottawa Scale52 and the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) checklist.53
The Grading of Recommendations Assessment, Development and Evaluation (GRADE)54 approach was used for rating the body of evidence into high, moderate, low, and very low quality. The quality of evidence for each outcome (risk factor) across all studies was rated according to a framework, including five factors that may lead to downgrading the quality of evidence and three factors that may lead to upgrading it.
Data synthesis
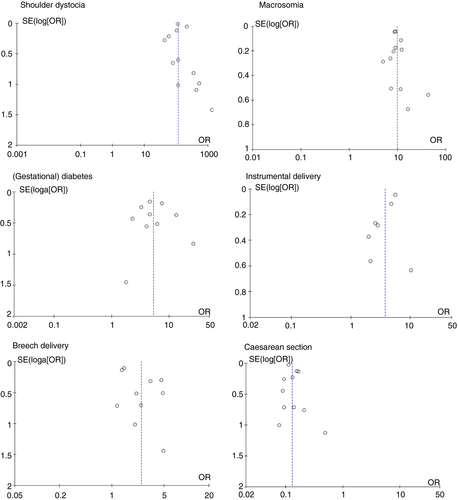
A first set of meta-analyses was conducted for each risk factor. The pooled odds ratio of NBPP was calculated with a 95% confidence interval.55 The weighing coefficients were computed by the Mantel–Haenszel method with a Dersimonian–Laird random effects model. Data analysis used Review Manager 5.3 software (RevMan; Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Where zeros caused problems with computation of the odds ratio or its standard error, 0.5 was added automatically in RevMan to all cells.56, 57 We used the I2 measure of Higgins and Green58 to assess across-study heterogeneity with I2 values of 0% to 40% considered to be low, 30% to 60% moderate, 50% to 90% substantial, and 75% to 100% considerable. This index does not depend upon the number of studies. If I2 was at least 50%, sensitivity analysis was performed by excluding the trials that potentially biased the results. Funnel plots, which are scatterplots of treatment effect (odds ratio of NBPP) against a measure of study precision (the standard error of the log[odds ratio]), were constructed to assess publication bias.59
In an additional meta-analysis, the pooled incidence of NBPP was calculated. The data from included studies were divided into corresponding timeframes: 1987 to 1995, 1996 to 2005, and 2006 to 2015. A pooled overall proportion of the event was calculated per timeframe, by a subgroup meta-analysis, using a Dersimonian–Laird random effects model with inverse variance, a logit transformation of the proportions, and Clopper–Pearson or ‘exact’ binomial confidence intervals for individual study results in the forest plot. The calculations were performed in R, version 3.5.2,60, 61 with the metaprop function in the meta library, version 4.9-4.
Results
Literature search
MEDLINE (974), Web of Science (747), ClinicalTrials.gov (0), and Embase (1215) provided a total of 2936 citations. After screening title and abstracts, 92 articles were included for full text review. Finally, 22 articles met the eligibility criteria. The main reasons for exclusion were study population (adult), outcome (other than risk factor), comparison group (no typically developing individuals), non-extractable data, and no full text available. Two articles62, 63 used an overlapping study population. The paper by Foad et al.63 was therefore excluded from the meta-analysis. The Cohen’s κ to evaluate concordance of independent reviewers was 0.70, indicating a substantial interrater agreement. A detailed PRISMA flow diagram is presented in Figure S2 (online supporting information).
Study characteristics
The characteristics of the 22 included articles are listed in Table S2 (online supporting information). Twelve were cohort studies62-73 and 10 were case–control studies.7, 15, 74-81 Only four studies had a prospective design.7, 15, 65, 70 Nine studies were conducted in Europe,7, 64-67, 72, 76-78 seven in North America,62, 63, 68, 69, 71, 75, 81 five in Asia,15, 70, 74, 79, 80 and one in Australia.73 The primary outcome in each study was risk factors for NBPP. In eight studies,62-64, 66, 68, 69, 75, 78 data were derived from nationwide databases whereas in 13 articles7, 15, 65, 70-74, 76, 77, 79-81 data originated from hospital records. Three studies linked clinical and administrative data sources from the hospital.71, 72, 80 The recruitment period of the studies varied from 5 months79 to 20 years,80 with a mean duration of 7 years 4 months and a standard deviation of 5 years 2 months. The sample size varied from 162 to 24 159 426 live births.
The historic risk factors for NBPP were listed (Table S3, online supporting information) and divided into maternal-, labour-, and fetal/neonatal-related factors. According to three studies,15, 72, 74 we added (para)medical-related as a new category.
The STROBE checklist (Table S4, online supporting information) showed an overall compliance of 67%. Matching criteria were mentioned in only 36 of the studies. Twenty-five per cent or less provided bias, sensitivity analyses, flow diagram, an explanation of how missing data were addressed, and the number of participants with missing data.
The methodological quality assessment of each study by the Newcastle-Ottawa Scale (Table S5, online supporting information) showed that 12 articles7, 15, 62-64, 66, 69-73, 78 achieved a score of at least seven stars, indicating high quality.82 The other 10 studies65, 67, 68, 74-77, 79-81 received at least five stars. Overall, study designs missed description of comparability.
Meta-analysis
Twenty-one studies represented a total sample size of 29 419 037. The five most frequently reported significant risk factors were used for meta-analysis: shoulder dystocia, birthweight, (gestational) diabetes, instrumental delivery, and breech delivery. Shoulder dystocia showed the highest association with NBPP (odds ratio [OR] 115.27; 95% confidence interval [CI] 81.35–163.35; 12 studies; n=25 825 074)7, 15, 62, 65, 71, 73, 75, 77-81 but with considerable across-study heterogeneity (I2=92%) (Fig. S3a, online supporting information). A sensitivity analysis with exclusion of three studies65, 71, 78 resulted in low heterogeneity (I2=19%; n=24 566 515) with similar strength of association (OR 113.58; 95% CI 95.35–135.29).
Macrosomia (≥4000g) was the second-highest associated factor (OR 9.75; 95% CI 8.29–11.46; 13 studies; n=2 435 212)15, 64, 67, 69-72, 74, 76-80 (Fig. S3b). The substantial heterogeneity (I2=70%) was reduced to 34% (n=2 955 694) by excluding two studies15, 70 and resulted in an odds ratio of 9.14% and 95% confidence interval 8.28 to 10.10. When subdividing the birthweight into three subcategories (4000–4499g, 4500–4999g, and ≥5000g), compared with normal birthweight of not more than 3999g, the odds ratios were respectively 6.32 (95% CI 5.48–7.29; six studies;64, 67, 72, 76-78 I2=59%), 20.77 (95% CI 16.86–25.58; five studies;64, 67, 72, 77, 78 I2=75%), and 55.21 (95% CI 49.7–61.35; five studies;64, 67, 72, 77, 78 I2=0%).
(Gestational) diabetes as a risk factor with an odds ratio of 5.33 (95% CI 3.77–7.55; I2=59%; n=1 651 281) was explored in 10 studies15, 65, 71, 72, 74, 75, 78-81 (Fig. S3c). Excluding two studies74, 75 reduced heterogeneity to 23% (n=1 274 770) and an odds ratio of 4.21 (95% CI 3.12–5.68).
With an odds ratio of 3.8 (95% CI 2.77–5.23; seven studies;7, 15, 67, 71, 72, 77, 78 I2=77%; n=1 849 398) instrumental delivery, containing vacuum and/or forceps delivery, occurred as a risk factor for NBPP (Fig. S3d). Sensitivity analysis affirmed three studies15, 67, 78 accountable, with an odds ratio of 2.50 (95% CI 1.81–3.45; n=25 240; I2=0%) after exclusion. For exploratory reasons a separate pooled odds ratio was calculated for vacuum (OR 6.03; 95% CI 3.61–10.07; seven studies;62, 65, 68, 75, 76, 79, 80 n=25 666 748; I2=96%) and forceps delivery (OR 4.97; 95% CI 1.95–12.66; three studies;62, 65, 68 n=25 284 297; I2=98%).
The last significant risk factor was breech delivery, with a pooled odds ratio of 2.49 (95% CI 1.67–3.7; 11 studies;7, 62, 63, 65-67, 72, 76-79 n=26 780 930; I2=70%) (Fig. S3e). The substantial heterogeneity reduced by excluding three studies62, 78, 79 (n=1 401 993; I2=0%), resulting in a higher odds ratio of 3.35 (95% CI 2.39–4.70).
Caesarean section emerged as a protective factor for NBPP, with a pooled odds ratio of 0.13 (95% CI 0.11–0.16; 11 studies;7, 62, 64, 65, 67, 68, 72, 74, 75, 77, 78 n=28 530 257; I2=41). Heterogeneity was moderate (Fig. S3f). The heterogeneity reduced to 0% by excluding one study62 (n=4 370 832; I2=0%; OR 0.15, 95% CI 0.13–0.17).
The five risk factors demonstrate an asymmetrical funnel plot, which could assume publication bias. The limited number of studies per risk factor, however, inhibits an appropriate causal interpretation (Fig. 1).59 According to the assessment by the GRADE (Table 1), Caesarean section presented high-quality evidence, shoulder dystocia, and birthweight moderate quality, whereas (gestational) diabetes and instrumental delivery showed low-quality evidence. Breech delivery had a very low confidence rating. Strengths of the studies responsible for upgrading were the large magnitude of effect. An important limitation leading to downgrading was inconsistency of results for each outcome and additionally imprecision for results of breech delivery. All findings are summarized in Table 2.
| Start | Downgrade | Upgrade | End | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Limitations (RoB) | Inconsistency of results | Indirectness of evidence | Imprecision | Publication bias | Large magnitude of an effect | Dose–response gradient | Effect of plausible residual cofounding | |||
| Shoulder dystocia | LQ | Low | ↓ −1 | OK | OK | OK | ↑ +2 | / | OK | MQ |
| (Gestational) diabetes | LQ | Low | ↓ −1 | OK | OK | OK | ↑ +1 | / | OK | LQ |
| Birthweight | LQ | Low | ↓ −1 | OK | OK | OK | ↑ +2 | / | OK | MQ |
| Instrumental delivery | LQ | Low | ↓ −1 | OK | OK | OK | ↑ +1 | / | OK | LQ |
| Breech delivery | LQ | Low | ↓ −1 | OK | ↓ −1 | OK | ↑ +1 | / | OK | VLQ |
| Caesarean section | LQ | Low | OK | OK | OK | OK | ↑ +2 | / | OK | HQ |
- RoB, Cochrane risk-of-bias tool for randomized studies; LQ, low quality; ↓, lower it down; OK, quality met criteria; ↑, grade it up; /, not applicable; MQ, moderate quality; VLQ, very low quality; HQ, high quality.
| Outcomea | Number of studiesb | Total population | Number of cases within risk group | Number of cases in total population | OR (95% CI) for NBPP | I2 (%) | Strength of recommendationc |
|---|---|---|---|---|---|---|---|
| Shoulder dystocia | 12 | 25 825 074 | 6563 | 33 984 | 115.27 (81.35–163.35) | 92 | Moderate |
| Macrosomia | 13 | 2 975 874 | 3896 | 5892 | 9.75 (8.29–11.46) | 70 | Low |
| (Gestational) diabetes | 10 | 1 651 281 | 175 | 3209 | 5.33 (3.77–7.55) | 59 | Moderate |
| Instrumental delivery | 7 | 1 849 398 | 824 | 3112 | 3.8 (2.77–5.23) | 77 | Low |
| Breech delivery | 11 | 26 780 930 | 183 | 34 960 | 2.49 (1.67–3.7) | 70 | Very low |
- a Risk factors implemented in the meta-analysis.
- b Included in the meta-analysis.
- c Based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) quality of evidence assessment. OR, odds ratio; CI, confidence interval; NBPP, neonatal brachial plexus palsy.
The pooled overall incidence of NBPP was 1.74 per 1000 live births (95% CI 1.56–1.94) (Fig. S4, online supporting information). The subgroup meta-analysis demonstrated a significant difference (p=0.013) in the pooled proportion of the event, between the three timeframes (1987–1995, 1996–2005, and 2006–2015). The forest plot showed highly heterogeneous individual study results within each timeframe. The estimated pooled proportions were 0.0021 (95% CI 0.0018–0.0025) for the first timeframe, 0.0019 (95% CI 0.0014–0.0025) for the second, and 0.0012 (95% CI 0.0009–0.0017) for the third. Although a considerable overlap was found between the first and second, and second and third, confidence intervals, a decrease in proportion could be concluded between the first and third timeframes.
Discussion
Consistent with previous research,83-86 shoulder dystocia was found to be the major risk factor for NBPP. Three studies were responsible for the considerable heterogeneity.65, 71, 78 First, we assigned the shoulder dystocia incidence variety as a possible source: shoulder dystocia is imprecisely coded, often underreported, and even unrecognized. The prospective design of Backe et al.65 and perhaps a more rigorous reporting of Backe et al.65 and Ouzounian et al.71 might explain their relatively high incidence of shoulder dystocia compared with previous published research. In contrast, the remarkably low incidence in the large retrospective case–control study of Mollberg et al.78 is explained by the authors as a possible underreporting of shoulder dystocia in Sweden. Second, an observable lower association of NBPP and shoulder dystocia was noted in two studies,60, 67 which could result from their immediate resort to the recommended shoulder dystocia delivery technique73, 87 in the case of shoulder dystocia.
Macrosomia, confirmed only after delivery of the neonate, is the second risk factor. The 70% heterogeneity was mostly caused by two studies.15, 70 As both were prospectively performed in Asia, their remarkably higher incidence of macrosomia might be explained by population bias. Sixty per cent of infants with macrosomia in the study by Najafian and Cheraghi70 were of Arab ethnicity. Dawodu et al.15 revealed a higher frequency of maternal diabetes, shoulder dystocia, and macrosomia in Arab versus Western populations. Both studies had a strong association of NBPP and macrosomia. Interestingly, only 2% of infants with macrosomia in the study by Najafian and Cheragi70 were associated with NBPP compared with 70% in that by Dawodu et al.15 Possibly, their birthweight distribution was different, with those in the study by Najafian and Cheragi70 tending to have the lower weight range: 85% birthweight between 4000 and 4449g, 13% between 4500 and 4999g, and only 2% having at least 5000g. Obstetric practices might also differ: all cases of NBPP in the study by Dawod et al.15 were delivered vaginally. Therefore, geographical differences, study design, and population bias might have been responsible for the heterogeneity. In agreement with previous studies,88-90 calculated odds ratios of NBPP expand powerfully with increasing birthweight: 6.32 for birthweight group 4000 to 4499g, 20.77 for 4500 to 4999g, and particularly 55.21 for those with a birthweight of at least 5000g. Birthweight of at least 5000g seems to be a commonly found risk factor, with I2=0%.
In accordance with previous reports,63, 89, 91-93 maternal diabetes is the third significant risk factor. Two studies,74, 75 showing higher odds for NBPP in the diabetic group, were responsible for the 59% heterogeneity. As the incidence of NBPP, respectively diabetes, in these studies was comparable with the included studies, other factors should be accountable. We believe that birthweight, ratio of head-to-abdominal circumference of the fetus, maternal weight, and age difference between diabetic versus non-diabetic groups are important characteristics possibly responsible for population bias. Disproportionate growth of trunk, measured as a smaller ratio of head circumference to abdominal circumference, is well-known in women with diabetes.94-96 Unfortunately, the included studies published none of these data. In the large population-based study by Freeman et al.,75 61% of diabetic mothers underwent Caesarean section, a finding also reported in other studies.97-99 The reason for Caesarean section was not mentioned but, knowing the diabetes diagnosis, the obstetrician may have preferred Caesarean section for fear of shoulder dystocia or NBPP.
Instrumental delivery (vacuum extraction and/or forceps delivery) augments the risk for NBPP 3.8-fold, with vacuum extraction showing an odds ratio of 6.03. The 77% heterogeneity was attributable to three studies.15, 67, 78 Different reporting numbers, depending on the use of maternal versus neonatal records, result in a probable source of bias. Instrumental delivery is often noted in the mother’s record as a procedure and only in the newborn infant’s record as a diagnosis if its use resulted in an injury. Possible population bias following different prevalence of other risk factors such as diabetes, macrosomia, shoulder dystocia, or increased length of second stage of labour in the studied population might cause heterogeneity. The different studies, despite reporting adjusted results, did not examine the same confounders. In addition, no information could be extracted on the sequential use of instruments, and certainly temporal trends are important.
Our meta-analysis confirms the previously reported NBPP risk of breech delivery.100-105 NBPP incidence and severity are increased with the tendency to develop more upper palsy and a higher percentage of bilateral palsy.106 Five out of 11 articles7, 65, 71, 76, 79 had a wide confidence interval, mainly because they included relatively few patients. Three studies were liable for the 70% heterogeneity.62, 78, 79 The contribution of Okby and Sheiner79 of 21% was possibly due to the presence of only one breech-delivered infant with NBPP. The low risk factor prevalence of 0.12% in the study by Abzug et al.62 might be explained by a higher rate of Caesarean section of 27%. In contrast, the study by Mollberg et al.78 presented 2.51% breech deliveries with a small complication rate, which could be ascribed to a more homogenous and better selection for trial of vaginal breech delivery.
Caesarean section seems to be a protective factor, with an odds ratio of 0.13. Low heterogeneity was reduced to 0% by excluding one study.62 The high prevalence of Caesarean section in the study by Abzug et al.62 might be the contributor.
The pooled overall incidence of NBPP was 1.74 per 1000 live births. The subgroup meta-analysis demonstrates a significant decrease of incidence over time (1987–1995, 1996–2005, and 2006–2015). The heterogeneity could be partly explained by the difference in type of obstetric care, in Caesarean section rate, and in average birthweight of neonates in different geographical regions.
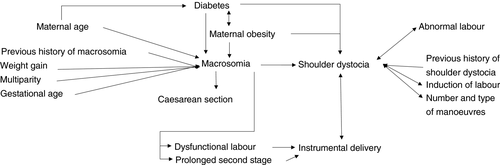
In practice, clinicians are confronted with a combination of different risk factors, which are interrelated (Fig. 2). The risk of shoulder dystocia is highly correlated with fetal macrosomia,75, 107 a history of previous shoulder dystocia7, 108 or macrosomia, maternal diabetes and obesity,109 induction of labour,73 abnormalities of labour, the number and type of manoeuvres used,73 and instrumental delivery.75, 110-112 Infants with macrosomia who are vaginally delivered are at risk of shoulder dystocia, with the highest association in the birthweight group greater than 5000g.78 Macrosomia is an important risk factor for instrumental delivery and increases the risk of Caesarean section. Most studies confirm the higher incidence of maternal obesity, macrosomia, disproportional fetal growth, abnormal labour, and shoulder dystocia among diabetic pregnancies.96, 113, 114 Other major risk factors for macrosomia are increased maternal age (>35y) and obesity, a positive history of previous macrosomia, prolonged pregnancy, and multiparity.64, 70, 113, 115, 116 Given the potential for severe neonatal and long-term morbidity of NBPP, as well as the significant medico-legal implications, it is essential to recognize patients who are at high risk (for instance by using a risk factor scoring system). However, prevention of NBPP is a challenge owing to the poor predictive value of the risk factors. Estimation of fetal weight, often used as a measure of risk stratification, remains, even with modern ultrasound equipment and in experienced hands, an inaccurate task.116 A sensitivity and specificity of respectively 60% and 90% for identifying fetuses of at least 4000g makes it insufficiently reliable.86, 90 Therefore, the target in antenatal prevention of NBPP should be on nutrition and physical activity guidance,117, 118 strict glycaemic and weight control in case of (gestational) diabetes, and appropriate counselling of women at high risk. Counselling should focus on the risks and alternatives to vaginal delivery.
Intrapartum risk management should include presence of consistent intrapartum guidelines, ensuring availability of regularly and well-trained staff at delivery and minimizing manoeuvres with excessive traction on the fetal neck. For macrosomia, recent research suggests that early-term induction significantly reduces the risk of shoulder dystocia.119-121 The clear association between NBPP and macrosomia in diabetic pregnancies, with higher occurrence of total palsies114 and therefore more permanent disability,116 is a major concern in the prevention of NBPP. Elective Caesarean section should be offered where there is an estimated fetal birthweight greater than 5000g in non-diabetic pregnancies90, 122 and greater than 4500g in those with diabetes according to guidelines.83, 90 In all others, a trial of labour is recommended. This requires preparedness for operative delivery, shoulder dystocia, and newborn asphyxia.114 In the case of shoulder dystocia, the risk for severity of NBPP is critically linked to a timely recognition, and increases with the number and types of manoeuvres required.73 Several authors123-127 reported evidence that a systematic approach with simulation training of specific manoeuvres can reduce cases of NBPP significantly.127, 128 For mothers with a previous shoulder dystocia history, an estimated fetal weight less than the previous dystocia delivery, or a lack of history of permanent NBPP, a trial of vaginal delivery may be reasonable.109
Independent or sequential use of forceps and vacuum extractor greatly increases the risk of NBPP, especially among obese and diabetic pregnancies.78, 129 The choice between vacuum and forceps has recently shifted, with a preference for vacuum as the instrument of first choice.130-133 Caesarean section performed after failed instrumental delivery carries increased risk.29 Therefore, obstetricians should aim to complete all operative vaginal delivery safely with a single instrument.134
The routine to deliver almost all term breech cases by elective Caesarean section is a continuing debate. In fact, there are only three randomized controlled trials that have investigated neonatal outcomes in term breech by mode of delivery.135-137 A Cochrane review138 reported a reduced perinatal or neonatal mortality among singleton infants delivered by a planned Caesarean section. However, neither elective Caesarean section nor vaginal delivery for breech presentation is risk free.105 Eligibility criteria, balancing all risks and benefits, for vaginal breech delivery should be set at the national level to guide best practice.
Although Caesarean section seems to be protective, it does not totally prevent NBPP68, 139 and carries significant neonatal and maternal morbidities (an increased risk for repeat Caesarean section, abnormal placentation, uterine rupture, and ectopic pregnancy).140, 141 This opens the commonly raised issue of the cost:benefit ratio.142-144 For the USA population, more than 1000 elective Caesarean sections, costing US$4 million to US$8 million, would be required to prevent one case of NBPP.75, 86 By comparison, the lifetime costs for a case of NBPP, excluding potential loss in productivity and earning capacity, is estimated at more than US$1 million.143 Caesarean section is only indicated in selected cases: women with previous children with permanent NBPP,23, 145 macrosomic pregnancies complicated by diabetes,74, 142, 143 and a high global risk identified by the obstetrician.
The decreasing incidence might be the result of augmented awareness of the problem and improved obstetric techniques and strategies. This encourages further research to determine predictable and modifiable risk factors. A future worldwide meta-analysis of incidence could be important to evaluate geographical differences and their influence on the risk of NBPP.
Strengths and limitations
The present study is, to our knowledge, the largest meta-analysis to investigate incidence and risk factors for NBPP with data obtained from 29 419 037 live births. The methodology used was rigorous, following the PRISMA statement. Baseline characteristics of the patients were largely comparable, which suggested that the population of patients was representative. The GRADE approach showed a high-quality body of evidence for Caesarean section, and moderate quality of evidence for shoulder dystocia and birthweight.
We recognize limitations. First, having investigated only the five most significant risk factors, we recommend further research for all previously mentioned, but also undiscovered, risk factors. Second, because most of the included studies were retrospective and observational by design, they were prone to bias. Specifically, some data might not have been accurately recorded or were underreported. Unclear definition of NBPP and its risk factors, but also increasing medical litigation related to birth trauma, might have contributed to reporting bias. Source bias (maternal vs neonatal, administrative vs medical, local hospitals vs national birth registers) was present. Nationwide databases are clearly more specific and include home deliveries. The divergent sample sizes caused different weight influence on the overall odds ratio. Temporal influences such as study duration and timing might contribute to imprecision. Third, as NBPP can be caused by inappropriate delivery technique, information about quality of delivery is necessary. Unfortunately, we could not assess to what extent poor obstetric technique contributed to heterogeneity. Fourth, we might have missed important information by excluding studies with non-extractable data and those solely investigating Erb palsy (defined as C5–6–[7]). Fifth, all included studies were from high-income countries, which may not be representative of all risk factors. Finally, lack of information about severity and duration of NBPP is a major limitation in stratifying the importance of each risk factor. Heterogeneity, important to the validity of conclusions, was therefore carefully analysed.
The additional incidence assessment is prone to potential selection bias as the search strategy was primarily intended for risk factors. Studies are restricted to four out of seven continents with therefore questionable generalizability of these data worldwide, where demographic variables may differ.
Conclusion
Shoulder dystocia, macrosomia, maternal diabetes, instrumental delivery, and breech delivery are the main risk factors for NBPP, with shoulder dystocia presenting the highest risk. Prevention remains difficult owing to the unpredictability of these factors and their often labour-relatedness. Moreover, many risk factors are interrelated. Caesarean section is a protective factor for NBPP. The incidence of NBPP has decreased over time, possibly as a result of increased awareness of the risk and improved obstetric strategies. In the view of its lifelong impact, the risk for NBPP, its severity, and morbidity should be further lowered. The focus needs to be on risk stratification, antenatal counselling, enhanced labour surveillance, and simulation training. This study highlights the need for further research to determine predictable and modifiable risk factors with emphasis on their relationship. As this meta-analysis integrates results of known risk factors, future research should also focus on undiscovered ones.
Acknowledgements
We thank Kristine Oostra and Wim Vanhove for their scientific support and sharing their clinical expertise. The authors have stated that they had no interest that could be perceived as posing a conflict or bias.




