Diagnosis and management of acute graft-versus-host disease
Summary
A joint working group established by the Haemato-oncology subgroup of the British Committee for Standards in Haematology (BCSH) and the British Society for Bone Marrow Transplantation (BSBMT) has reviewed the available literature and made recommendations for the diagnosis and management of acute graft-versus-host disease. This guideline includes recommendations for the diagnosis and grading of acute graft-versus-host disease as well as primary treatment and options for patients with steroid-refractory disease. The goal of treatment should be effective control of graft-versus-host disease while minimizing risk of toxicity and relapse.
Summary of Recommendations
- An accountable transplant physician should be responsible for supervising the treatment of patients with acute graft-versus-host disease (GvHD) (1C).
- Clinical criteria should define acute GvHD and not purely time post-transplant (1B).
- Clinical diagnosis is appropriate if the classical constellation of symptoms is present. Biopsies may be helpful if the diagnosis is unclear but should not delay management (1A).
- At diagnosis, the extent of individual organ involvement and overall grade of acute GvHD should be documented, taking into account all organ involvement, as this has prognostic significance (1A).
- The modified Seattle Glucksberg criteria (Przepiorka et al 1995) are recommended for grading (1A).
- The management of grade 1 disease should include topical therapy and optimizing levels of calcineurin inhibitors without the need for additional systemic immunosuppression (1C).
- The use of systemic corticosteroids is recommended for first line therapy for grade II–IV GvHD (1A).
- Two milligram/kg per day of methylprednisolone is recommended as the starting dose for patients with grades III–IV GvHD (1A).
- One milligram/kg per day of methylprednisolone is recommended for patients with grade II GvHD (2B).
- The use of ‘nonabsorbable’ steroids can be considered for acute intestinal GvHD in order to reduce the dose of systemic steroids (2B).
- The following agents are suggested for use in the second line treatment of steroid-refractory acute GvHD: extracorporeal photopheresis, anti-tumour necrosis factor α antibodies, mammalian target of rapamycin (mTOR) inhibitors, mycophenolate mofetil, interleukin-2 receptor antibodies (2C).
- The following agents are suggested as third line treatment options in acute steroid-refractory GvHD: alemtuzumab, pentostatin, mesenchymal stem cells and methotrexate (2C).
Introduction
Graft-versus-host disease (GvHD) remains a major complication of haematopoietic stem cell transplantation. The incidence of acute GvHD (aGvHD) is 10–80%, depending upon the risk factors present (Sullivan, 2004). Corticosteroids are effective in <50% of patients (Martin et al, 1990). A number of novel agents have been developed and investigated, both as first line treatments and for management of steroid-refractory disease. Despite these developments, there is no consensus on the optimal strategy for managing GvHD.
A joint working group established by the Haemato-oncology subgroup of the British Committee for Standards in Haematology (BCSH) and the British Society of Blood and Marrow Transplantation (BSBMT) has reviewed the available literature and made recommendations for the diagnosis and management of GvHD. The guideline has been split into three documents, including ‘Diagnosis and Management of Acute GvHD’ (present guideline), ‘Diagnosis and Management of Chronic GvHD’ (Dignan et al, 2012a) and ‘Organ-specific Management and Supportive Care in GvHD’ (Dignan et al, 2012b). These guidelines are designed to be used together and to complement each other in order to provide an evidence-based approach to managing this complex disorder. The goal of treatment should be effective control of GvHD while minimizing the risk of toxicity and relapse. The prevention of aGvHD using prophylactic agents will not be included in the current document. These guidelines discuss GvHD following allogeneic transplant and will not discuss transfusion-related GvHD.
- Diagnosis
- Grading
- First line treatment
- Second line treatment
- Third line treatment
- Other agents
- Organ-Specific Management of GvHD:
- Cutaneous
- Gastrointestinal
- Genital
- Liver
- Ocular
- Oral
- Pulmonary
- Infection prophylaxis in GvHD
- Infection prophylaxis in GvHD
- Vaccinations
- Management of complications associated with long-term steroid use
Methodology
- Establishment of a working group comprising experts in the field of allogeneic transplantation followed by literature review to 17 June 2011 including Medline, internet searches and major conference reports.
- Development of key recommendations based on randomized, controlled trial evidence. Due to the paucity of randomized studies some recommendations are based on literature review and a consensus of expert opinion.
- The GRADE nomenclature was used to evaluate levels of evidence and to assess the strength of recommendations. The GRADE criteria are specified in the BCSH guideline pack and the GRADE working group website (see Appendix I). Further information is available from the following websites:
- http://www.bcshguidelines.com/4_HAEMATOLOGY_GUIDELINES.html
- http://www.gradeworkinggroup.org/index.htm
- Review by the BCSH committees, BSBMT executive committee, the UK Photopheresis Society and the UK Paediatric Bone Marrow Transplant Group.
- Review by sounding board of the British Society for Haematology (BSH) and allogeneic transplant centres in the UK.
Children
- A substantial number of children undergo transplants for non-malignant disorders and will not benefit from the graft-versus-malignancy effect. This may have implications for the choice of GvHD therapy.
- There may be a variety of co-morbidities due to the underlying disease which may alter the appearance of GvHD.
- The toxicities of treatment may be different in a growing child with a developing organ system and these side effects should be considered when choosing a treatment option
Diagnosis
The diagnosis of aGvHD is predominantly based on clinical findings and is often one of exclusion. Any form of aGvHD can be associated with culture-negative fever. The skin is the organ most commonly involved at the onset of aGvHD followed by gastrointestinal (GI) tract and liver (Martin et al, 1990). Skin GvHD typically causes a maculopapular rash, which usually starts on the palms and soles but may commence in any part of the skin. It then usually spreads to include the rest of the body. In the most severe forms a reaction similar to toxic epidermal necrolysis may occur, with widespread skin involvement and mucocutaneous ulceration and bullae (Vogelsang et al, 2003). The differential diagnosis includes drug reactions, viral exanthems, engraftment syndrome and effects of chemotherapy or radiation. The distribution of the rash over the face, palms and soles is more common in aGvHD compared to drug rashes and the association with hyperbilirubinaemia and diarrhoea also makes GvHD more likely (Byun et al, 2011).
Cutaneous biopsy may be important, particularly in the absence of the classical constellation of symptoms. Histological features include apoptosis at base of epidermal rete pegs, dyskeratosis, exocytosis of lymphocytes, satellite lymphocytes adjacent to dyskeratotic epidermal keratinocytes and perivascular lymphocytic infiltration in the dermis (Ferrara & Deeg, 1991; Goker et al, 2001). Cutaneous biopsy was not found to be useful in predicting severity of disease: a retrospective study of 51 skin biopsies showed that there was poor correlation between the clinical severity of skin rash and biopsy findings and concluded that skin biopsies may have a limited role in the management of aGvHD (Zhou et al, 2000). A decision analysis (Firoz et al, 2006) also concluded that, in the allogeneic stem cell transplantation group, the best outcomes would be obtained with treatment of GvHD and no skin biopsy. More recently, elafin has been found to be overexpressed in GvHD skin biopsies. Plasma levels of elafin have been found to be significantly higher at the onset of skin GvHD, and correlate with severe GvHD, with a greater risk of death relative to other known risk factors (hazard ratio, 1.78) and may have prognostic value as a biomarker of skin GvHD (Paczesny et al, 2010). Carefully designed prospective studies with blinded pathological review and careful assessment of clinical outcomes are necessary to confirm the test characteristics of skin biopsy in aGvHD. Biopsies may be helpful where the diagnosis is unclear but should not delay management in patients with classical clinical features of aGvHD.
GI aGvHD typically leads to secretory diarrhoea but nausea, vomiting, anorexia, weight loss and abdominal pain can also occur. Diarrhoea can be copious and, in severe aGvHD, bleeding may result from mucosal ulceration (Nevo et al, 1999) and ileus may ensue. The differential diagnosis includes the side effects of chemotherapy or other drugs and infection of the GI tract. Patchy involvement can lead to a normal appearance on endoscopy (Ponec et al, 1999) but a positive correlation has been demonstrated between endoscopic findings from intestinal mucosa and histological grading (Cruz-Correa et al, 2002). Biopsies taken at endoscopy may show patchy ulcerations, apoptotic bodies at crypt bases, crypt ulceration and flattening of surface epithelium (Snover et al, 1985). In patients with symptoms suggestive of upper GI involvement with aGvHD, upper GI endoscopy as well as lower endoscopic biopsy may be helpful (Roy et al, 1991). In patients who underwent both upper and lower GI investigation, however, biopsy of the rectosigmoid was found to be the most informative in a retrospective study of 112 patients (Ross et al, 2008). Imaging of the GI tract may reveal luminal dilatation, thickening of the small bowel wall or air or fluid levels suggestive of an ileus (Vogelsang et al, 2003). Positron emission tomography may also be helpful in the assessment of intestinal GvHD (Stelljes et al, 2008). Biopsies may be helpful where the diagnosis is unclear but should not delay management in patients with classical clinical features of aGvHD. Biopsies may be particularly helpful to exclude alternative or co-existing GI pathologies or in patients who do not respond quickly to first line treatment.
Liver aGvHD typically presents with jaundice and a cholestatic pattern of liver injury including elevated conjugated bilirubin, alkaline phosphatase and gamma-glutamyltranspeptidase. A hepatitic picture with elevation of alanine aminotransferase has been described in aGvHD occurring after donor lymphocyte infusion as well as in some patients with acute and chronic GvHD (Strasser et al, 2000; Fujii et al, 2001; Akpek et al, 2002). This pattern of liver function derangement is particularly variable in children. Total bile acids and cholesterol in the serum are also elevated, while coagulopathy and hyperammonaemia develop in more severe cases. Serum lipoprotein X, a nonspecific marker of persistent cholestasis, can become positive during evolution to chronic GvHD (Zidan et al, 2008). Additional clinical signs of liver aGvHD are painful hepatomegaly, dark urine/pale stools and fluid retention. Nonspecific symptoms, such as fever, lack of appetite and nausea, are common. Pruritus can follow in more severe forms. The differential diagnosis is wide and includes veno-occlusive disease of the liver, viral infections, drug toxicity and sepsis.
Histopathological findings can include endothelialitis, lymphocytic infiltration of the portal areas, pericholangitis and bile-duct destruction (Snover et al, 1984). Duarte et al (2005) also suggested that high levels of lobular inflammation and a low level of hepatocyte ballooning were independent favourable prognostic factors for non-relapse mortality. Iron overload, frequently described in the liver biopsies, is often a sequel of previous blood product requirements rather than a pathogenic element in aGvHD, but could represent an adverse prognostic factor (Oshrine et al, 2011). In practice, it is often difficult to perform liver biopsies due to the bleeding risks associated with thrombocytopenia (Ferrara et al, 2009). A recent paediatric study reported complications following 5 out of 18 liver biopsies in haematopoietic stem cell transplant patients, including four patients who had undergone transjugular biopsy (Oshrine et al, 2011).
Historically, aGvHD has been described as occurring before 100 d post-transplant, with hyperacute GvHD occurring before neutrophil engraftment. The revised National Institute for Health (NIH) criteria now define classic aGvHD as occurring before 100 d and late onset aGvHD which has typical signs and symptoms but occurs after 100 d (Filipovich et al, 2005). An overlap syndrome has also been described where patients have features of both acute and chronic GvHD. Both late onset and overlap syndrome arise more frequently after reduced-intensity conditioning (Filipovich et al, 2005).
Recommendations
- An accountable transplant physician should be responsible for supervising the treatment of patients with acute GvHD (1C).
- Clinical criteria should define acute GvHD and not purely time post-transplant (1B).
- Clinical diagnosis is appropriate if the classical constellation of symptoms is present. Biopsies may be helpful if diagnosis is unclear but should not delay management (1A).
Grading
The first grading criteria for aGvHD were published Glucksberg et al (1974). These criteria include a stage between 1 and 4 for each organ involved, which are then combined to give an overall grade, from I to IV. A subsequent grading system was devised following a consensus conference in 1994, which removed the need to assess clinical performance (Przepiorka et al, 1995; reviewed in Devergie, 2008). These grading systems include the percentage of skin involvement. The percentage of body surface area involved can be calculated using the ‘rule of nines’ (Hettiaratchy & Papini, 2004). A further staging system was defined by the International Bone Marrow Transplant Registry (IBMTR), which classified severity from A to D based on transplant outcome (Rowlings et al, 1997).
A prospective study was undertaken to compare the Glucksberg criteria with those from the IBMTR (Cahn et al, 2005). This study failed to show a clear benefit of one system over the other. There was less physician bias in assigning grades with the IBMTR scoring system but the Glucksberg system was better at predicting early survival (Cahn et al, 2005). In practice, most centres in the United Kingdom still use the modified Glucksberg criteria based on the outcome of the consensus conference in 1994 because data collection for the European Bone Marrow Transplant (EBMT) registry is based on this system.
The grade of aGvHD has been shown to correlate with overall survival. The Chronic Leukaemia Working Group of the EBMT reviewed 1294 patients receiving an allogeneic transplant for chronic myeloid leukaemia (Gratwohl et al, 1995). The transplant-related mortality (TRM) for grades 0–IV aGvHD was 28%, 27%, 43%, 68% and 92%, respectively. A distinct difference in prognosis was noted between grades 0–1 and grades II–IV (Gratwohl et al, 1995).
Recommendations
- At diagnosis, the extent of individual organ involvement and overall grade of aGvHD should be documented, taking into account all organ involvement, as this has prognostic significance (1A).
- The modified Seattle Glucksberg criteria (Przepiorka et al , 1995 ) are recommended for grading (1A).
Management of acute GvHD
Overview
There are few reports of randomized controlled trials of the management of aGvHD and patients should be included in trial protocols wherever possible. Second and third line options are likely to be determined on availability of treatments, financial considerations and individual patient and physician preferences.
Management of grade I disease
Patients with grade I disease are not likely to require systemic treatment. Cutaneous aGvHD may respond to topical steroid creams. Suitable strengths of topical steroids are detailed in Table 1. Antihistamines may be helpful in patients with pruritis. In resistant grade I aGvHD, topical tacrolimus may also be useful although this is not a licensed indication. Adults should commence on 0·1% tacrolimus until resolution. If skin flares on stopping, 0·03% tacrolimus may be of benefit to aid weaning. 0·03% tacrolimus may be used in children aged 2–15 years. Patients should be reviewed frequently for other organ manifestations of GvHD and worsening of skin rash.
| Steroid strength | Very potent e.g. Dermovate | Potent e.g. Betnovate | Moderately potent e.g. Eumovate | Mildly potent e.g. 1% Hydrocortisone |
|---|---|---|---|---|
| Face | Should generally be avoided |
Twice daily 4–12 weeks |
Twice daily 6–12 months |
Twice daily Long term use acceptable |
| Body |
Twice daily 4–12 weeks |
Twice daily Long term therapy may be appropriate |
||
| Palms and soles |
Twice daily May be used under occlusion to enhance efficacy. Long term therapy may be appropriate |
Twice daily Long term therapy may be appropriate |
- Generally a lower steroid usage is recommended in children.
- Dermatology supervision is advised when using potent steroids on the face.
- Side effects: prolonged use of topical steroids can thin skin and may cause erythema, striae and dyspigmentation. If more than 50 g of very potent steroid is used per week, sufficient steroid may be absorbed through the skin to result in adrenal gland suppression or Cushingoid features. Occlusion of steroids will enhance absorption and it is possible that larger amounts of weaker steroids may have the same effect.
Calcineurin inhibitors are commonly used in the prophylaxis of GvHD. In patients where levels are sub-therapeutic the dose should be adjusted to ensure a therapeutic level.
Recommendation
- The management of grade I disease should include topical therapy and optimizing levels of calcineurin inhibitors without the need for additional systemic immunosuppression (1C).
First line treatment of grade II–IV disease
An algorithm summarizing first line treatment of aGvHD is shown in Fig 1.
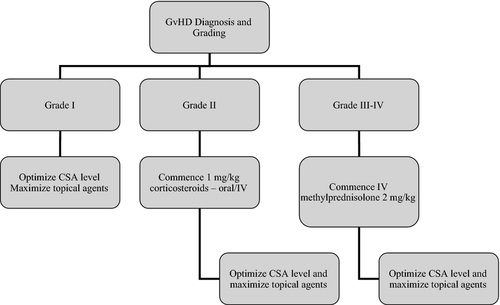
Calcineurin inhibitors
Patients with grade II–IV disease are likely to require additional systemic agents to achieve a response. As in the management of grade I disease, in patients who are already receiving calcineurin inhibitors, the dose should be adjusted to ensure a therapeutic level. In patients who are no longer receiving calcineurin inhibitors, physicians may consider restarting these agents at a therapeutic dose. In those already receiving ciclosporin, switching to tacrolimus was only found to be helpful in those patients with neurotoxicity (Furlong et al, 2000).
Corticosteroids
Corticosteroids have been used as the standard first line treatment for aGvHD for several decades. Their effect is likely to be due to lympholytic effects and anti-inflammatory properties (Deeg, 2007). Two early studies assessed response to corticosteroids in patients with grade II–IV GvHD (Martin et al, 1990; Weisdorf et al, 1990). Martin et al (1990) reported overall complete or partial responses in 44% of patients. Improvement rates were 43% for skin disease, 35% for evaluable liver disease and 50% for evaluable gut disease. Weisdorf et al (1990) reported a complete and continued resolution of aGvHD in 41% of patients after a median of 21 d of corticosteroids. MacMillan et al (2002a) reported a retrospective study of 443 patients who received prednisolone 60 mg/m2 for 14 d followed by an 8-week taper. A complete response was observed in 35% of patients and a partial response in 20% of patients (MacMillan et al, 2002a). Corticosteroids are also the mainstay of treatment for patients with hyperacute GvHD (Deeg, 2007; Saliba et al, 2007).
It has also been shown that TRM is higher in patients who do not respond to steroids. Weisdorf et al (1990) reported a 5-year survival of 51% for steroid responders compared to 32% for steroid non-responders (P = 0·004). A similar increase in TRM was reported by Martin et al (1990) in those patients who did not achieve a complete response to steroids. In addition, an early response seems to be indicative of improved long-term outcome. Van Lint et al (2006) reported a higher TRM and worse survival in those that had not responded after 5 d of methylprednisolone.
There is no consensus on the optimal dose and duration of steroid therapy due to the paucity of available literature. Van Lint et al (2006) suggested that 5 d of steroid therapy is sufficient to identify those patients who are responding to treatment and who may be able to start tapering their dose while Cragg et al (2000) continued treatment for 7 d. An IBMTR survey confirmed that most centres considered patients to be steroid-refractory after 5 d of treatment (Hsu et al, 2001). Similarly, this survey demonstrated that most centres used 2 mg/kg per day of methylprednisolone to be the standard primary dose. In general, intestinal and liver aGvHD require more prolonged steroid therapy than skin disease although response times vary significantly between patients (Deeg, 2007).
A recent report retrospectively compared a dose of 1 mg/kg per day (prednisolone-equivalent dose) to 2 mg/kg per day of (prednisolone-equivalent dose) and found no difference in patient outcomes in those with grade I/II disease. Definitive conclusions could not be drawn for those patients with grade III or IV disease due to the small numbers in this group (Mielcarek et al, 2009).
One prospective randomized trial compared a short versus long prednisolone taper. Patients in the long taper group achieved resolution of aGvHD after a median of 30 d of therapy compared to 42 d in the short taper group. There was no difference in survival, incidence of chronic GVHD or steroid-related complications between the two groups (Hings et al, 1993).
A prospective, randomized trial comparing 2 mg/kg per day of methylprednisolone with 10 mg/kg per day of methylprednisolone showed no advantage of high-dose steroids. The transplant mortality was 30% in both groups (Van Lint et al, 1998). The use of methylprednisolone at doses higher than 2 mg/kg per day is not routinely recommended and would be at the discretion of the treating physician.
‘Nonabsorbable’ steroids
Budesonide and beclomethasone have both been used in the treatment of GI GvHD. The role of nonabsorbable steroids has recently been summarized (reviewed in Ibrahim et al, 2009). Budesonide was used in combination with systemic corticosteroids in 22 patients with acute intestinal GvHD. A response rate of 70% was reported compared to 33% in an historical control group (P < 0·01) (Bertz et al, 1999). There have been two subsequent randomized placebo controlled studies of beclomethasone diproprionate and systemic corticosteroids compared to systemic corticosteroids alone. McDonald et al (1998) reported a significantly better response in patients who received beclomethasone diproprionate and systemic corticosteroids compared to systemic corticosteroids alone (McDonald et al, 1998). The second trial showed a cumulative rate of GvHD-treatment failure of 31% for the combination arm compared to 48% for the control arm (P = 0·12) (Hockenbery et al, 2007). This study and two others demonstrated the potential of nonabsorbable steroids to reduce the dose of systemic steroids (Bertz et al, 1999; Iyer et al, 2005). Nonabsorbable steroids may be beneficial in providing an increase in response and a steroid-sparing effect in patients with aGvHD. The authors recognize that although the term ‘nonabsorbable’ is widely used it is possible that a small amount of the drug may be systemically absorbed.
Recommendations
- The use of systemic corticosteroids is recommended for first line therapy for grade II–IV GvHD (1A).
- Two milligram/kg per day of methylprednisolone is recommended as the starting dose for patients with grades III–IV GvHD (1A).
- One milligram/kg per day of methylprednisolone is recommended for patients with grade II GvHD (2B).
- The use of ‘nonabsorbable’ steroids can be considered for acute intestinal GvHD in order to reduce the dose of systemic steroids (2B).
Second line treatment
The addition of second line agents can be considered in patients who have failed to respond despite 2 mg/kg of IV methylprednisolone in conjunction with a calcineurin inhibitor for 5 d or progressive symptoms after 72 h (Deeg, 2007). Unfortunately, the available evidence for efficacy of individual agents is often derived from small trials and is frequently contradictory. Second line options may include extracorporeal photopheresis (ECP), mycophenolate mofetil (MMF), anti-tumour necrosis factor α (anti-TNF) antibodies or mammalian target of rapamycin (mTOR) inhibitors. An algorithm detailing the second line treatment of grade III–IV GvHD is shown in Fig 2. Patients who fail one-second line agent should generally try another second line agent before moving on to third line options.
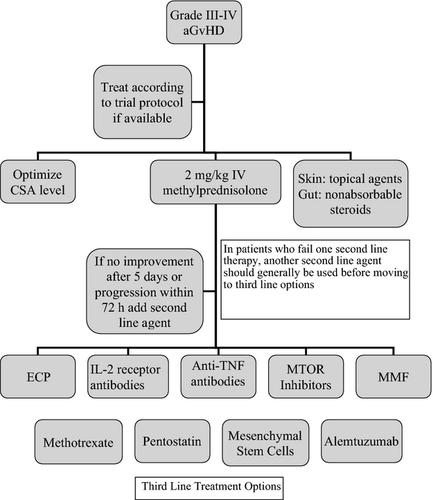
Extracorporeal photopheresis
ECP is a cell-based immune-modulatory therapy that offers a different therapeutic approach. ECP involves processing up to 15% of the patients total blood volume per cycle, isolating a buffy coat (approx 5 × 109 leucocytes) and adding 8-methoxypsoralen followed by ultraviolet-A (UVA) irradiation before it is returned to the patient. The mode of action of ECP in GvHD is the focus of active research and may be multifactorial in nature (reviewed in Marshall, 2006). ECP induces apoptosis of all leucocytes (including activated T-cells) within 24 h of return. The reinfusion of these cells and subsequent phagocytosis by antigen presenting cells (APCs) may regulate immune homeostasis through modulation of cytokine production and tolerance induction of APCs (Bladon & Taylor, 2006; Perritt, 2006).
There are fewer reports detailing the role of ECP in aGvHD compared to chronic GvHD. The initial reports included small patient numbers but did suggest efficacy of ECP in the acute setting (Smith et al, 1998; reviewed in Dall'Amico & Messina, 2002). A retrospective series of 23 patients with acute steroid-refractory GvHD reported a complete response rate of 52% although no patients with grade IV GvHD had a complete response (Perfetti et al, 2008). A trend for improved survival was seen in grade III/IV GvHD compared to matched controls (38% vs 16%; P = 0·08) (Perfetti et al, 2008). The largest published series to date was a Phase 2 prospective study, which included 59 patients with steroid-refractory or steroid-dependent GvHD treated with two consecutive ECP treatments every week (Greinix et al, 2006). Complete responses were reported in 82% of patients with cutaneous involvement, 61% of liver involvement and 61% with gut involvement (Greinix et al, 2006). At 4 years, TRM was 36%. The use of ECP in the treatment of aGvHD in the UK has been reported by Das-Gupta et al (2011). In a series of 19 patients with steroid-refractory aGvHD, 11 patients showed a clinical response including 5/10 with grade IV GvHD (Das-Gupta et al, 2011).
Positive results have also been reported in children treated with ECP. Perotti et al (2010) reported a response rate of 68% in 50 children treated with ECP for aGvHD. The standard UVAR XTS™ (Therakos, Ascot, UK) machine is only suitable for children over 40 kg in weight although the newer CELLEX™ (Therakos, Ascot, UK) machine is now available, which allows treatment of patients < 40 kg.
ECP has an excellent safety profile. The side effects appear to be mild and include hypotension, fevers and reduced haemoglobin level (Greinix et al, 1998; Perotti et al, 1999). There are no reports of increased infection risk or disease relapse. An indwelling catheter is required in patients with poor venous access. At present, access to ECP for aGvHD in the UK is generally limited to those centres where ECP is available on site as patients are often too unwell to travel for treatment. The optimal treatment schedule and duration of treatment has yet to be established. Das-Gupta et al (2011) reported a regimen of weekly cycles for a minimum of 8 weeks continued until maximal response or complete response seen.
Anti-TNF antibodies
Several studies have investigated the role of blocking the inflammatory cytokine tumour necrosis factor α (TNFα). TNFα is involved in the pathophysiology of GvHD by activating APCs, recruiting effector cells and causing direct tissue damage (Reddy & Ferrara, 2003). Earlier animal models had suggested that TNF played a major role in aGvHD of GI tract and skin (Hattori et al, 1998). Reports have investigated both the role of infliximab and etanercept.
Infliximab is an anti-TNFα monoclonal antibody. Several small case series suggested a possible benefit of infliximab in the treatment of steroid-refractory GvHD (Hervé et al, 1992; Kobbe et al, 2001; Couriel et al, 2004; Patriarca et al, 2004). There are also reports of an increased risk of infection in patients treated with infliximab (Marty et al, 2003). In a larger study of 52 patients (71% of whom had grade III/IV GvHD), 15% achieved a complete response with infliximab as salvage therapy (Pidala et al, 2009). In addition, a Phase 3 study of 63 patients comparing infliximab plus corticosteroids to corticosteroids alone in aGvHD did not show any improvement in response rate or overall survival in patients with newly diagnosed aGvHD (Couriel et al, 2009).
Etanercept is a soluble dimeric TNFα receptor 2, which renders TNFα inactive by competing for binding sites (Sieper & Van Den Brande, 2005). The drug is administered subcutaneously and has a good side effect profile (Sieper & Van Den Brande, 2005). Etanercept has been used in several studies in the primary treatment of aGvHD. A pilot study reported a 75% response rate in 20 patients with grade II/III aGvHD treated with etanercept and methylprednisolone (Uberti et al, 2005). A further Phase 2 study was reported by the same group comparing etanercept plus methylprednisolone in 61 patients (20 of whom had been included in the pilot study) compared to a contemporaneous group of 99 patients who received steroids alone for initial treatment of aGvHD (Levine et al, 2008). Patients treated with etanercept were more likely to achieve a complete response than those treated with steroids alone (69% vs 33%, P < 0·001) (Levine et al, 2008). Busca et al (2007) reported a response in 6/13 patients with refractory gut GvHD. Both of these studies suggested that the GI tract was particularly sensitive to TNF blockade. The infection rate was not significantly different between the two populations. Two-thirds of the patients had grade II disease.
Sirolimus (Rapamycin)
Sirolimus (Rapamycin) exerts its immunosuppressive effect through inhibition of mTOR. In a pilot trial, Benito et al (2001) treated 21 patients with steroid-refractory grade III/IV GvHD with sirolimus and reported response rates of 57%. The drug was discontinued in 10 patients due to lack of improvement in GvHD, myelosuppression or seizures. Five patients developed haemolytic uraemic syndrome, particularly when sirolimus was combined with calcineurin inhibitors, although the incidence of this complication in the absence of calcineurin inhibitors is very low (Benito et al, 2001). Similar response rates were observed in a retrospective study of 22 patients and in a recent report of 34 patients treated with sirolimus in combination with corticosteroids (Ghez et al, 2009; Hoda et al, 2010). There is also one study of 32 patients using sirolimus as primary treatment. Durable complete remissions were reported in 16 patients (Pidala et al, 2011).
Sirolimus may be helpful as a second line treatment option in acute GvHD. In view of the risk of haemolytic uraemic syndrome and hyperlipidaemia, mTOR inhibitors should be used with caution in combination with calcineurin inhibitors. mTOR inhibitors also have a number of other drug interactions and a dose reduction may be required when used in combination with certain drugs including all azole antifungal agents. When used in combination with azoles, the initial dose of sirolimus should be reduced by 40–50%. Regular monitoring of drug levels is recommended to avoid toxicities. Sirolimus has a half-life of about 70 h so daily levels are not required and monitoring drug levels 2–3 times per week, even after dose modification, is recommended. A sirolimus level of 4–8 ng/ml is recommended (Wolff et al, 2011). The lipid profile must be monitored on a monthly basis during treatment with mTOR inhibitors. Lipid lowering medication may be required. Intravenous preparations of alternative mTOR inhibitors have been used in acute GvHD but small numbers of patients and high toxicities prevent any recommendations on their use.
Mycophenolate mofetil
MMF is the 2-(4-morpholino) ethyl ester of mycophenolic acid (MPA). MMF is rapidly absorbed following oral administration and hydrolysed to MPA. MPA blocks the de novo pathway of purine synthesis in lymphocytes by selectively and reversibly inhibiting inosine monophosphate dehydrogenase (Allison & Eugui, 2000). Several small series have suggested a role of MMF in the management of refractory aGvHD as a steroid-sparing agent (Basara et al, 2001; Krejci et al, 2005; Takami et al, 2006; Furlong et al, 2009). One study reported a response in a small group of patients but noted a high level of life-threatening infectious complications (Onishi et al, 2010).
In one arm of a randomized Phase 2 study MMF was administered with corticosteroids and compared to etanercept, pentostatin and denileukin diftitox in the initial management of aGvHD. Patients treated with MMF had highest day 28 complete response rates, overall survival (64%) and lowest infection rates compared to the other agents (Alousi et al, 2009). These results should be interpreted with caution as the patients who received the other agents had already failed prophylaxis with MMF so may have represented a higher risk group. In addition, it should be noted that this series was not powered to compare across the study arms and that 68% of patients had grade I/II GvHD.
Interleukin 2 receptor antibodies
The interleukin-2 (IL-2) receptor alpha subunit (CD25) is predominantly expressed on activated T lymphocytes and has been a particular target for monoclonal antibody treatment for GvHD. Daclizumab is a humanized immunoglobulin G1 monoclonal antibody that binds to the alpha subunit of the high-affinity IL-2 receptor and inhibits IL-2 binding. Several studies reported promising results in steroid-refractory patients but a randomized controlled trial using daclizumab with corticosteroids as upfront therapy (Lee et al, 2004) showed significantly worse survival compared to corticosteroids alone (Anasetti et al, 1994; Przepiorka et al, 2000; Lee et al, 2004; Bordigoni et al, 2006). Daclizumab is no longer commercially available and therefore cannot be recommended for use in aGvHD.
Other drugs targeting the CD25 subunit have been developed. Denileukin diftitox links amino acids of the diphtheria toxin to amino acids of IL-2 in an attempt to enhance therapeutic efficacy. Two small studies showed promising results in steroid-refractory disease (Ho et al, 2004; Shaughnessy et al, 2005) but this agent is no longer available for use in the UK.
Other IL-2 receptor antibodies have been used. The use of inolimomab, a murine anti-IL-2R was retrospectively evaluated 85 patients with steroid-refractory aGvHD (Bay et al, 2005). The total response rate was 63% and overall survival at a median follow-up of 20 months was 26%. A further retrospective study of 40 patients reported a 58% response rate with higher responses in those without GI disease (Piñana et al, 2006).
Basiliximab is a chimeric IL-2 receptor antagonist. A small Phase 1 study of 17 patients reported a 71% response rate in patients with steroid-refractory GvHD and 53% survival at a median of 157 d post-transplant (Massenkeil et al, 2002). A prospective Phase 2 study of 23 patients reported an overall response rate of 82.5% (Schmidt-Hieber et al, 2005). Several other small studies have reported similar efficacy (Funke et al, 2006; Wang et al, 2008; Liu et al, 2009). A larger study recently reported a response in 46/53 patients with steroid-refractory aGvHD. Twenty eight patients were alive at a median follow-up of 16 months (Wang et al, 2011a).
Rao et al (2009) reported on 22 children who received daclizumab in combination with infliximab for steroid-refractory GvHD; 19/22 patients responded. Survival at a median follow up of 31 months was 68% and there were only two infection-related deaths (Rao et al, 2009). A Phase 2 study using daclizumab in combination with etanercept in 21 patients reported a clinical response in 14 patients but 11 died of infection-related complications (Wolff et al, 2005). Srinivasan et al (2004) used daclizumab [alone or with infliximab/antithymocyte globulin (ATG)] with comprehensive infection prophylaxis in 12 patients and showed improved survival compared to an historical control group. A recent study treated 17 steroid-refractory aGvHD patients with combination daclizumab and infliximab; clinical response was observed in 47% of patients but all died at a median of 35 d from initiation of treatment (Rager et al, 2011). As daclizumab is no longer commercially available this combination cannot be recommended at present.
Recommendation
- The following agents are suggested for use in the second line treatment of steroid-refractory acute GvHD: extracorporeal photopheresis, anti-tumour necrosis factor a antibodies, mammalian target of rapamycin (mTOR) inhibitors, mycophenolate mofetil, interleukin-2 receptor antibodies (2C).
Third line treatment
A number of other agents have been investigated for the treatment of steroid-refractory disease. In patients who fail a second line treatment option another second line option should generally be considered before moving to a third line treatment option. Some agents may be used in combination but there is little data to support this approach. The agents that may be considered for third line treatment options are discussed below and shown in Fig 2. These agents are considered to be third line options as there is less evidence available for their use. The authors acknowledge that some of these agents have not been studied in the context of third line treatment of acute GvHD.
Mesenchymal stem cells
Mesenchymal (stromal) stem cells (MSCs) are a population of undifferentiated pluripotent stem cells that modulate immune and inflammatory response and facilitate repair of connective tissues (Pittenger et al, 1999; Majumdar et al, 2000). Le Blanc et al (2004) were the first to report the efficacy of MSCs for the treatment of aGvHD. The same group subsequently undertook a Phase 2 study of MSCs in patients with refractory GvHD (Le Blanc et al, 2008). This report included 55 patients (25 children, 30 adults) with steroid-resistant, severe aGvHD. Thirty patients had a complete response and nine showed improvement. Overall survival at 2 years post-transplant was 53% in complete responders compared to 16% in those who did not respond (Le Blanc et al, 2008). There were no significant adverse events. An encouraging report by Karlsson et al (2008) suggests that MSCs have little effect on T-cell responses to Epstein–Barr virus and cytomegalovirus (CMV), despite their strong immunosuppressive effects on alloreactive T-cells.
Prochymal® (Genzyme, Cambridge, UK) MSCs have been used as part of a compassionate use programme. In 12 children with grade III or IV gut GvHD a complete response was seen in seven patients and 5/12 were alive after a median follow-up of 611 d (Prasad et al, 2011). The same commercially generated MSCs have recently been used in a multicentre randomized controlled trial and reported in abstract form (Martin et al, 2010). One hundred and sixty-three patients received MSCs and 81 received placebo. Although this study did not show improved complete response rates overall in steroid-refractory aGvHD compared to the control arm, patients with steroid-refractory gut and liver aGvHD showed significantly improved response rates (82% and 76%, respectively) (Martin et al, 2010). Furthermore, it should be noted that not all sources of MSCs are equivalent.
A recent report used MSCs for the primary treatment of aGvHD in combination with corticosteroids (Kebriaei et al, 2009). Thirty-one evaluable patients were included and randomized to receive a dose of either 2 or 8 × 106 MSCs/kg. Seventy-seven percent of patients had a complete response rate and 16% had a partial response rate. There were no differences in safety or efficacy between the two groups (Kebriaei et al, 2009). Some success has also been reported using MSCs expanded in vitro with human serum (Pérez-Simon et al, 2011). This study included 10 adult patients with acute refractory GvHD and demonstrated a complete response in one patient, a partial response in six patients and no response in the remaining three patients.
MSCs are currently available in the UK for paediatric patients with steroid-refractory aGvHD as part of the Prochymal® compassionate use programme from Genzyme. In addition, for both adults and children MSCs may be available from Imperial College (Professor Francesco Dazzi). A randomized study using MSC in upfront therapy of grade 3–4 aGvHD is also planned. MSCs are a promising treatment in the management of acute GvHD. At present, the authors suggest that MSCs may be considered as a third line treatment option but recognize that this is an area of active research and that MSCs may have a greater role in the management of aGvHD in the future.
Alemtuzumab
Alemtuzumab (Campath 1H) is a humanized, unconjugated IgG1 kappa monoclonal antibody that is specific for CD52 receptors present on mature T and B lymphocytes, monocytes, monocyte-derived dendritic cells, macrophages and eosinophils (Hale, 2001). Several case reports suggested that alemtuzumab might be helpful in the management of aGvHD (Varadi et al, 1996; Carella et al, 2004; Wandroo et al, 2004). In a prospective study, 18 patients with steroid-refractory aGvHD received alemtuzumab 10 mg subcutaneously once daily for 5 d. At day 28, 83% had responded to alemtuzumab and 10/15 of responders were alive after a median follow up of 11 months. Infectious complications were reported in 14 patients, including CMV reactivation in 11 patients (Gómez-Almaguer et al, 2008). In a series of 20 patients with histologically confirmed grade III/IV steroid-refractory GvHD, the overall response rate was 70% and 1-year overall survival was 50% (Schnitzler et al, 2009). These results have not been replicated in all studies. In a Phase 2 trial of 10 patients, five patients responded but all died within a median of 40 d of treatment (Martínez et al, 2009). These studies were predominantly undertaken in patients who had not received T-cell depletion prior to transplantation and it is possible that the effect may be different in T-cell depleted patients.
Pentostatin
Pentostatin is a nucleoside analogue that is a potent inhibitor of adenosine deaminase. Cell death occurs as a result of accumulation of 2-deoxyadenosine 5-triphosphate, particularly in T-cells and Natural Killer (NK) cells. The drug also causes reduced TNFα and prolonged lymphopenia (Margolis & Vogelsang, 2000; Foss, 2006). It has been used in the treatment of both aGvHD and chronic GvHD. A Phase 1 study of 23 evaluable patients found the maximum tolerated dose to be 1.5 mg/m2 per day for 3 d. Fourteen patients achieved a complete response but median survival was 85 d (Bolaños-Meade et al, 2005). A small retrospective series including 12 patients with aGvHD reported overall response in 6/12 patients but median survival of only 1.4 months (Pidala et al, 2010). Pentostatin was also used in combination with corticosteroids in one arm of a randomized Phase 2 study for initial therapy of aGvHD comparing etanercept, MMF and denileukin diftitox. The day-28 complete response rate was 38%, which was lower than MMF (60%) and denileukin diftitox (53%). Overall survival at 9 months was 47%, which was similar to denileukin diftitox and etanercept but lower than MMF (64%). The infection rate of 57% was also higher compared to MMF (44%) and etanercept (48%) (Alousi et al, 2009). A recent study including 23 patients with steroid-refractory aGvHD reported an 83% response rate with a 2-year survival rate of 43% (Klein et al, 2011).
Methotrexate
Methotrexate is commonly used in GvHD prophylaxis, but there is less evidence for its use in the management of aGvHD. Huang et al (2005) reported a response in 18/19 steroid-refractory patients treated with low dose methotrexate. Wang et al (2011b) used low dose methotrexate combined with low dose methylprednisolone as a first line treatment in 32 patients with aGvHD and reported an overall response rate of 81%. De Lavallade et al (2006) reported a response in 7/12 patients with refractory aGvHD. A paediatric study reported a response rate in 7/10 paediatric patients with steroid-dependent or steroid-refractory disease (Inagaki et al, 2008).
Recommendation
- The following agents are suggested as third line treatment options in acute steroid-refractory GvHD: alemtuzumab, pentostatin, mesenchymal stem cells and methotrexate (2C).
Agents with a possible role in the management of acute GvHD
Several other agents have been used in the management of acute GvHD. These are discussed below.
Antithymocyte globulin (ATG)
MacMillan et al (2002b) reported some efficacy of ATG in steroid-refractory patients in a retrospective review but this has not been reported by all investigators (Roy et al, 1992; Arai et al, 2002). In a prospective study, Cragg et al (2000) compared equine ATG with steroids to steroids alone as initial treatment for aGvHD. Complete and partial response rates of 76% were reported in both groups. More patients experienced infectious complications in the combination arm compared to those receiving steroids alone and there was a trend towards improved survival in the prednisolone arm (Cragg et al, 2000). Similar results were reported by Van Lint et al (2006), who treated 211 patients with grade I–IV GvHD with 2 mg/kg per day of methylprednisolone for 5 d. The non-responders were then randomized to receive either a higher dose of steroids of 5 mg/kg per day for 10 d either alone or in combination with rabbit ATG. 26% of patients achieved a complete response but there was no significant difference between the two groups in terms of response rate, TRM and survival (Van Lint et al, 2006). The authors recognize that many centres have experience in using ATG for the treatment of acute GvHD but, in view of the lack of evidence supporting its use, suggest that the use of ATG is at the discretion of the treating physician.
Regulatory T-cells
Trzonkowski et al (2009) reported a transient response in one patient with aGvHD treated with human ex vivo expanded CD4+CD25+CD127− T regulatory cells. The use of regulatory T-cells in the management of aGvHD remains experimental at present (Trzonkowski et al, 2009).
Agents not currently recommended in the management of acute GvHD
Rituximab is an anti-CD20 monoclonal antibody that has been used in the treatment of chronic GvHD but its role in aGvHD is limited to case reports (Kamble et al, 2006). The role of visilizumab, a monoclonal antibody directed at the invariant CD3 epsilon chain of the T-cell receptor, and the anti-CD147 antibody, ABX-CBL have been used in the treatment of aGvHD but are not currently available (reviewed in Deeg, 2007).
Various other agents have been tried in the management of GvHD. These include thalidomide and azathioprine. Thalidomide was not found to be effective in the management of aGvHD (Kulkarni et al, 2003). There is no evidence to support the use of azathioprine in this context.
Intra-arterial methylprednisolone has been used in the management of steroid-refractory aGvHD but at present there is insufficient experience to recommend this technique (Weintraub et al, 2010; Milner et al, 2011).
Disclaimer
While the advice and information in these guidelines is believed to be true and accurate at the time of going to press, neither the authors, the British Society for Haematology, the British Society of Blood and Marrow Transplantation nor the publishers accept any legal responsibility for the content of these guidelines.
Acknowledgements
The authors are grateful to the BCSH task force and BSBMT executive committee for their support in preparing these guidelines.
Author contributions
FLD reviewed the literature and wrote the initial draft of the manuscript. MNP chaired the writing group, reviewed the literature and revised the manuscript. AC represented BCSH, reviewed the literature and revised the manuscript. GJ represented BSBMT, reviewed the literature and revised the manuscript. JS, JC, PA, PT, PM, NH and BES reviewed the literature and revised the manuscript.
Conflicts of interest
All authors have declared any potential conflicts of interest to BCSH. FLD and BES have received research funding, honoraria and speaker__s fees from Therakos, a Johnson and Johnson company. PCT has received honoraria from Therakos, a Johnson and Johnson company. MNP has participated in an advisory board for EUSA Pharma SAS. None of the other authors have declared any conflicts of interest.
Appendix 1: GRADE nomenclature for assessing levels of evidence and providing recommendations in guidelines
Strength of recommendations
Strong (grade 1): Strong recommendations (grade 1) are made when there is confidence that the benefits do or do not outweigh harm and burden. Grade 1 recommendations can be applied uniformly to most patients. Regard as ‘recommend’.
Weak (grade 2): Where the magnitude of benefit or not is less certain a weaker grade 2 recommendation is made. Grade 2 recommendations require judicious application to individual patients. Regard as ‘suggest’.
Quality of evidence
- High: Further research is very unlikely to change confidence in the estimate of effect. Current evidence derived from randomized clinical trials without important limitations.
- Moderate: Further research may well have an important impact on confidence in the estimate of effect and may change the estimate. Current evidence derived from randomized clinical trials with important limitations (e.g. inconsistent results, imprecision – wide confidence intervals or methodological flaws – e.g. lack of blinding, large losses to follow up, failure to adhere to intention to treat analysis),or very strong evidence from observational studies or case series (e.g. large or very large and consistent estimates of the magnitude of a treatment effect or demonstration of a dose-response gradient).
- Low: Further research is likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. Current evidence from observational studies, case series or just opinion.