Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas
Summary
In June 2005, an ad hoc Expert Committee formed by the Pituitary Society convened during the 9th International Pituitary Congress in San Diego, California. Members of this committee consisted of invited international experts in the field, and included endocrinologists and neurosurgeons with recognized expertise in the management of prolactinomas. Discussions were held that included all interested participants to the Congress and resulted in formulation of these guidelines, which represent the current recommendations on the diagnosis and management of prolactinomas based upon comprehensive analysis and synthesis of all available data.
Definition
Prolactinomas are pituitary adenomas that express and secrete prolactin (PRL) to variable degrees, are almost invariably benign, but are nevertheless frequently clinically significant and may be challenging to manage. Prolactinomas are generally classified according to size as microadenomas (less than 10 mm in diameter) or macroadenomas (more than 10 mm in diameter). Over 90% of prolactinomas are small, intrasellar tumours that rarely increase in size.1 Occasionally, these adenomas can be aggressive or locally invasive and cause compression of vital structures. Malignant prolactinomas that are resistant to treatment and disseminate inside and outside the central nervous system (CNS) are very rare.
It is not known how prolactinomas develop, but the process may involve an early genome mutation that results in a mutated pituitary stem cell. Various permissive factors may then stimulate the proliferation of these mutated cells. Familial prolactinomas have also been described2 suggesting that a genetic component might contribute to the pathogenesis.
Endocrine features
Prolactinomas contain lactotrophs that secrete PRL, a process that is enhanced by oestrogen and inhibited by dopamine, which is synthesized by the hypothalamus and transported to the pituitary by portal vessels. Prolactinomas lead to hyperprolactinaemia, but drugs or situations that inhibit hypothalamic production of dopamine, its transport to the pituitary, or its effectiveness at dopaminergic receptors can also cause hyperprolactinaemia without prolactinoma. Therefore, hyperprolactinaemia does not invariably indicate concomitant prolactinoma. Although oestrogen stimulates prolactin secretion, there is no evidence linking oestrogen therapy to the formation of prolactinomas.1,3,4 Mixed growth hormone and PRL-secreting tumours are well recognized, and usually give rise to acromegaly in association with hyperprolactinaemia. PRL-secreting adenomas may also produce thyroid-stimulating hormone (TSH) or adenocorticotrophic hormone (ACTH), but such tumours are uncommon.1,3 Occasionally, prolactinomas may be a component of multiple endocrine neoplasia syndrome type I (MEN I)5 although this occurs too infrequently to justify screening for MEN I in every patient with a prolactinoma, a basal measurement of calcium is advisable.
Epidemiology and clinical presentation
Epidemiology
About 40% of all pituitary adenomas are prolactinomas. Age prevalence varies widely, and prolactinomas have been reported in patients from 2 to 80 years. Prolactinomas are more common in women, with a peak incidence during the childbearing years. Although these tumours often come to attention after the discontinuation of an oral contraceptive there is no correlation between oral contraceptive use and the development of prolactinomas.
Clinical features
The clinical features of a prolactinoma predominantly result from hyperprolactinaemia. Prolactin stimulates milk production but also has secondary effects on gonadal function, and the clinical features of hyperprolactinaemia mostly result from the effects of PRL on gonadal activity. Hyperprolactinaemia interrupts the pulsatile secretion of gonadotrophin-releasing hormone, inhibits the release of luteinizing hormone and follicle-stimulating hormone, and directly impairs gonadal steroidogenesis. Collectively, these actions lead to various forms of primary (in children), or secondary amenorrhoea.
In very large tumours, compression of other pituitary cells or of the hypothalamic-pituitary stalk can cause hypopituitarism. Neurological manifestations are common in patients with macroadenomas or giant adenomas, because they are space-occupying lesions and with possible compression of the optic chiasm. Neurological symptoms include headaches, visual impairment ranging from quadrantanopia to classical bitemporal hemianopia or scotomas. Blindness due to an expanding prolactinoma is an exceptional event, but may occur in the setting of pituitary apoplexy.
Men
In men, hyperprolactinaemia usually causes impotence, infertility and decreased libido. Men commonly present with larger tumours and neurological symptoms. This may be due to delayed recognition of symptoms or because of biological differences in tumour growth.
Women
Most prolactinomas in women are microadenomas. Approximately 90% of premenopausal women present with oligo/amenorrhoea, and up to 80% also exhibit galactorrhoea and may also manifest anovulatory infertility. Postmenopausal women with hyperprolactinaemia do not present with these classical symptoms and are often only recognized when a large adenoma produces mass effects, such as headache or visual disturbances. However, under oestrogen replacement therapy, hyperprolactinaemic postmenopausal women may experience galactorrhoea.
Chronic hyperprolactinaemia-induced hypogonadism is associated with reduced spinal bone mineral density in both sexes, but an increased incidence of pathological fractures in women with hyperprolactinaemia has not been so far reported.6,7 After prolactin normalization, bone mineral density increases but does not always return to normal.7
Children
Prolactinomas are uncommon in children, but when they occur, there appears to be a greater proportion of macroadenomas compared to adults. Presenting symptoms include delayed puberty in both sexes, and primary amenorrhoea and galactorrhoea in girls. In boys the presentation is similar to what occurs in adult men. Because of the increased prevalence of macroadenomas, prolactinomas in children are more frequently accompanied by neurological symptoms.8
Causes of hyperprolactinaemia other than prolactinoma
Craniopharyngioma and other sellar or parasellar masses,9 granulomatous infiltration of the hypothalamus, head trauma, and large pituitary adenomas other than prolactinomas can all cause hyperprolactinaemia secondary to either impairment of hypothalamic production of dopamine or a compression of the pituitary stalk that impairs dopamine transport to the pituitary. Prolactin levels can also be elevated in patients with chronic renal or hepatic failure, usually due to decreased clearance of PRL. The polycystic ovarian syndrome is commonly associated with elevated PRL levels as well.10 Some patients with primary hypothyroidism have mild hyperprolactinaemia. It has been proposed that this is due to the increased synthesis of, or sensitivity to, hypothalamic TRH, which is able to stimulate pituitary lactotroph cells, but the true cause is unknown. In cases of primary hypothyroidism with thyrotroph cell hyperplasia, care should be taken to distinguish pituitary enlargement from prolactinoma. During pregnancy there is a progressive increase in prolactin to levels as high as 10 times normal because of pituitary lactotroph hyperplasia induced by the high oestrogen levels secreted by the placenta. PRL levels can also rise modestly after exercise, meals, stress, chest wall stimulation, or sexual intercourse. Finally, idiopathic hyperprolactinaemia needs to be considered as a diagnosis.11
Drug effects
Pharmacological agents that reduce dopamine secretion or action can cause hyperprolactinaemia. These drugs include metoclopramide, phenothiazines, butyrophenones, risperidone, serotonin-re-uptake inhibitors (rare), sulpiride, domperidone and verapamil (Table 1).12 In a patient with mild hyperprolactinaemia who is taking a psychoactive drug, verapamil, or oestrogen, the drug is probably responsible.
| Medication class | Medication types/examples |
|---|---|
| Antipsychotics/ neuroleptics | Phenothiazines |
| Butyrophenones | |
| Atypical antipsychotics | |
| Antidepressants | Tricyclic and tetracyclic antidepressants |
| Monoamine oxidase (MAO) inhibitors | |
| Selective serotonin re-uptake inhibitors (SSRI) | |
| Other | |
| Opiates | |
| Cocaine | |
| Antihypertensive medications | Verapamil |
| Methyldopa | |
| Reserpine | |
| Gastrointestinal medications | Metoclopramide |
| Domperidone | |
| H2 blockers? | |
| Protease inhibitors? | |
| Oestrogens | |
- * Adapted from Molitch 2005.12
Diagnosis of prolactinomas
Laboratory findings
The diagnosis of prolactinoma requires both radiographic evidence of pituitary adenoma and laboratory analyses documenting the presence of sustained hyperprolactinaemia. Normal prolactin levels in women and men are below 25 µg/l and 20 µg/l, respectively, with the more commonly used assays (1 µg/l is equivalent to 21·2 mIU/l, WHO Standard 84/500). However, other assays may yield PRL values that are lower or higher; accordingly, the normal range should be adjusted for the specific assay used. Increases in PRL due to interference with dopamine action are usually modest, with levels rarely exceeding 150 µg/l (∼3000 mIU/l).1,3
In general, serum prolactin levels parallel tumour size. PRL values between the upper limits of normal and 100 µg/l (∼2000 mIU/l) may be due to psychoactive drugs, oestrogen, or functional (idiopathic) causes, but can also be caused by microprolactinomas. Most patients with PRL levels over 150 µg/l (∼3000 mIU/l) (five times higher than the normal values) will have a prolactinoma. Macroadenomas are typically associated with levels of over 250 µg/l (∼5000 mIU/l), and in some cases over 1000 µg/l (∼20000 mIU/l). Nevertheless such values are not absolute; prolactinomas can present with variable elevations in PRL, and there may be dissociation between tumour mass and hormonal secretion. Therefore, caution must be exercised when interpretating a moderate elevation of PRL in a patient with a pituitary macroadenoma, because the cause of the hyperprolactinaemia may be compression of the pituitary stalk by a tumour other than a prolactinoma.1,3,11
Clinical assessment
The recommended diagnostic evaluation for prolactinomas is summarized in Fig. 1. When evaluating a patient with symptoms consistent with hyperprolactinaemia and persistently elevated serum PRL, secondary causes should first be ruled out by a careful clinical history, physical examination, pregnancy test, routine biochemical analysis (to evaluate kidney and liver function), and TSH determination. If the patient is taking a drug known to cause hyperprolactinaemia, it is important to determine if the drug is indeed the cause by withdrawing the drug for at least 72 h, if this can be done safely. It is essential to discuss the issue with the prescribing physician or to obtain a psychiatric evaluation before stopping any psychiatric drugs suspected of causing hyperprolactinaemia. If feasible, the patient could be switched to an alternative drug that does not cause hyperprolactinaemia (Table 2). When the drug cannot be stopped, particularly in a patient with neurological symptoms, the evaluation should include magnetic resonance imaging (MRI) of the sella to exclude a mass lesion.
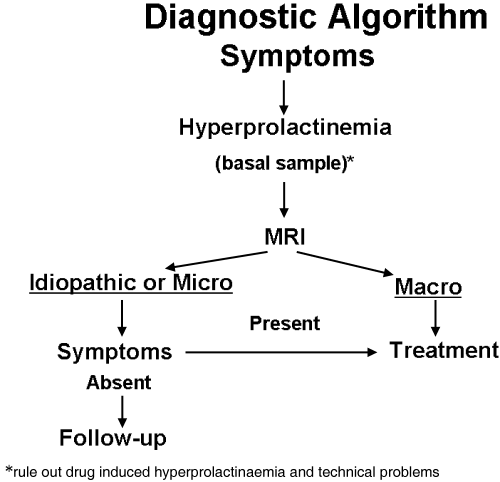
Recommended diagnostic algorithm for prolactinomas.
| Antipsychotics | PRL increase | Antidepressants | PRL increase |
|---|---|---|---|
| Typical | Tricyclics | ||
| phenothiazines | + + + | amitryptyline | + |
| butyrophenones | + + + | desipramine | + |
| Atypical | chlomipramine | + + + | |
| risperidone | + + + | nortriptyline | – |
| molindone | + + | imipramine | CR |
| clozapine | 0 | maprotiline | CR |
| quetiapine | + | amoxapine | CR |
| ziprasidone | 0 | MAO Inhibitors | |
| aripiprazole | 0 | ||
| olanzapine | + | ||
| pargyline | + + + | ||
| clorgyline | + + + | ||
| tranylcypromine | ± | ||
| SSRIs | |||
| fluoxetine | CR | ||
| paroxetine | ± | ||
| citalopram | ± | ||
| fluvoxamine | ± | ||
| Other | |||
| nefazodone | 0 | ||
| bupropion | 0 | ||
| venlaflaxine | 0 | ||
| trazodone | 0 |
- 0, no effect; ±, minimal increase but not to abnormal levels; +, increase to abnormal levels in a small percentage of patients; ++, increase to abnormal levels in 25–50% of patients; +++, increase to abnormal levels in > 50% of patients.
- CR, isolated case reports of hyperprolactinaemia but generally no increase in prolactin levels; MAO, monoamine oxidase; SSRIs, selective serotonin re-uptake inhibitors.
- * Adapted from Molitch 2005.12
The initial determination to establish hyperprolactinaemia must avoid excessive venipuncture stress and ideally should be performed at least 1 h after awakening or eating. A single measurement of PRL is usually adequate for diagnosis. When initial PRL values are not diagnostic (for example, above the normal laboratory range, but not high enough to clearly indicate a prolactinoma), sampling should be repeated on another day. In this case, to avoid the effect of pulsatile secretion, two to three samples separated by at least 15–20 min should be obtained. Pregnancy is not always known or communicated by the female patient with hyperprolactinaemia, and therefore a pregnancy test should be obtained to rule out pregnancy-associated hyperprolactinaemia before continuing with the diagnostic evaluation.
Patients with large tumours should be screened for hypopituitarism. Some groups routinely perform initial basal determinations of all pituitary and peripheral hormones plus IGF-I13 to rule out associated hypersecretion, and also to establish baseline reference values for comparison following future interventions.
Diagnostic pitfalls: macroprolactin and the ‘hook effect’
There are two potential pitfalls in the diagnosis of a prolactinoma: the presence of macroprolactin and the so-called ‘hook effect’. Macroprolactin is a complex of PRL and, generally, an IgG antibody. Serum PRL concentrations are elevated secondary to a reduced rate of clearance of this complex. Macroprolactin has reduced bioactivity and is present in significant amounts in up to 20% of hyperprolactinemic sera, resulting in pseudo-hyperprolactinaemia and potential misdiagnosis. Macroprolactin is detected by most but not all PRL assays; therefore each centre must know the specific characteristics of the prolactin immunoassay they use. For confirmation of macroprolactinaemia, polyethylene glycol precipitation is the most practical method. Alternatively, size exclusion chromatography can be used, but is time-consuming and not suitable for routine use.14 Whether macroprolactin should be measured in patients with ‘classic’ symptoms is controversial. However, it is reasonable to ascertain the presence of macroprolactin in patients with moderately elevated PRL levels (25–150 µg/l: 500–3000 mIU/l) and less typical symptoms, such as headaches or diminished libido in the presence of regular menses.15
The ‘hook effect’ may be observed when the serum PRL concentration is extremely high, as in some cases of giant prolactinomas. The high amount of circulating PRL causes antibody saturation in the immunoradiometric assay, leading to artifactually low results. To overcome the hook effect, an immunoradiometric PRL assay should be performed at a serum dilution at 1 : 100, or alternatively should include a washout between the binding to the first antigen and the second step in order to eliminate excess unbound PRL. It has been recommended that the hook effect be excluded in all new patients with large pituitary macroadenomas who have normal or mildly elevated PRL levels.16,17
Dynamic tests of prolactin secretion
Several dynamic tests of PRL secretion have been proposed as diagnostic tools in the evaluation of hyperprolactinaemia, including administration of TRH, l-dopa, nomifensine, domperidone, and insulin-induced hypoglycaemia. Despite the fact that some of these methods are useful in particular hands,18 it is now widely accepted that the diagnosis of a prolactinoma should be confirmed by analysing basal PRL values, imaging the pituitary, and excluding other causes as outlined above.18,19
Pituitary imaging
Confirmation of the diagnosis of a prolactinoma requires not just laboratory evidence of sustained hyperprolactinaemia, but also radiographic evidence of a pituitary adenoma. After excluding potential secondary causes of hyperprolactinaemia including pregnancy, a gadolinium-enhanced MRI should be performed. Computed tomography (CT) with intravenous contrast enhancement is less effective than MRI in diagnosing small adenomas and in defining the extension of large tumours, but may be used if MRI is unavailable or contraindicated. It should be noted that microadenomas are present in about 10% of the normal population. Also, a normal MRI examination does not necessarily exclude a microadenoma.19 Patients with macroadenomas that abut the optic chiasm should undergo formal visual-field examination (e.g. computerized Goldman perimetry), but visual testing is not necessary in patients with microadenomas.
Hyperprolactinaemia in the presence of an MRI-detected pituitary adenoma is consistent with but not unequivocally diagnostic of a prolactinoma, because any pituitary mass that compresses the pituitary stalk may cause hyperprolactinaemia. Unequivocal diagnosis requires pathological analysis, but prolactinomas are rarely surgically removed. As an alternative, the empirical confirmation of the diagnosis can be obtained by pharmacological treatment for several months with dopamine agonists with serial assessment of serum PRL levels and adenoma size. Three outcomes are possible following a course of therapy. Normalization of PRL plus a substantial reduction (75% or more) of the initial adenoma size confirms the diagnosis of a prolactinoma. Normalization of PRL with no change or only a small reduction in tumour volume may suggest a pituitary adenoma other than a prolactinoma. No change in serum PRL and no reduction in tumour volume indicate a resistant prolactinoma.20
Medical treatment of prolactinomas
Who should be treated?
The therapeutic approach proposed for prolactinomas is shown schematically in Fig. 2. The primary goal of therapy in patients with microprolactinomas is to restore gonadal and sexual function by normalizing PRL levels, but in patients with macroadenomas, control and reduction of tumour size are also important.
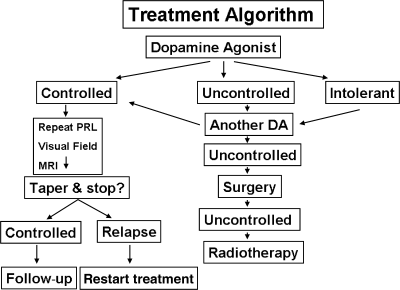
Recommended treatment algorithm for prolactinomas.
All patients with macroadenomas and most patients with microprolactinoma require treatment. Indications for treatment include infertility, a pituitary tumour with neurological effects (particularly visual defects), bothersome galactorrhoea, long-standing hypogonadism, alterations in pubertal development, and prevention of bone loss in women because of hypogonadism. Occasionally, patients with mild hyperprolactinaemia with regular menses who wish to become pregnant may also require treatment. Premenopausal women with normal menstrual cycles and tolerable galactorrhoea, and postmenopausal women with tolerable galactorrhoea who have idiopathic hyperprolactinaemia or microprolactinoma, should be reassured and not actively treated. However, such patients must be carefully followed with periodic PRL measurements to detect potential enlarging tumours.1,3,11
Pharmacological options
Dopaminergic agonists such as bromocriptine and cabergoline are the primary therapy for patients with hyperprolactinaemia and prolactinomas. These drugs normalize PRL levels and significantly reduce the volume of the tumour in most patients, and extensive experience has demonstrated their utility in treating prolactinomas of all sizes.21,22 All dopamine agonists are efficacious, but pergolide and quinagolide are less commonly used. Patients who are resistant to, or who cannot tolerate a particular dopamine agonist, should be switched to an alternative dopamine agonist.23
Large comparative studies of cabergoline and bromocriptine have convincingly demonstrated the superiority of cabergoline in terms of patient tolerability and convenience, reduction in prolactin secretion, restoration of gonadal function, and decrease in tumour volume.24–26 Cabergoline is effective in most patients, including those who did not previously respond to bromocriptine.23 Nevertheless, bromocriptine has been used satisfactorily for years21 and since it is less expensive it should be considered in medical settings with limited budgets.
Therapy with bromocriptine (tablet of 2·5 mg) is initiated with a dose of 0·625–1·25 mg daily and increased by 1·25 mg at weekly intervals until a dose generally of 2·5 mg twice or thrice daily is reached. Side-effects such as upper gastrointestinal disturbances and postural hypotension can be reduced by using an incremental dosage schedule and taking tablets with a snack before retiring. Cabergoline (tablet of 0·5 mg) therapy is begun at a dose of 0·25–0·5 mg administered once or twice weekly, and the dose is increased monthly until prolactin secretion normalizes.3,26 Doses over 3 mg per week are rarely necessary. Hyperprolactinaemia will prove self-limiting in up to one-third of women, and in others pregnancy may induce a return to normal PRL function.27,28 Women with hyperprolactinaemia who pass through menopause may normalize their prolactin levels and therefore in such women, reassessment of the need for continuing treatment of hyperprolactinaemia is indicated.29
For patients with medication-induced hyperprolactinaemia, the primary treatment is to stop the drug or to switch to an alternative drug, but oestrogen/testosterone replacement therapy could also be considered. Dopamine agonist therapy is generally not recommended in such situations, and when hyperprolactinaemia is caused by psychoactive drugs, treatment with dopamine agonists should be considered only after careful psychiatric counselling.
Treatment of microprolactinomas
The primary clinical objective in treating microprolactinomas is to restore gonadal function and fertility, and medical therapy is remarkably effective in achieving these goals. In 90–95% of cases, microadenomas do not progressively increase in size, so suppression of tumour growth is not a treatment goal.28,30 Microadenomas often shrink and sometimes disappear during long-term effective dopamine agonist treatment. Once normal PRL levels are achieved, annual serial assessments of PRL should be performed. If the PRL levels do not normalize, changing to an alternative dopamine agonist may be effective.3 It should be noted, however, that in some patients dopamine agonists can normalize gonadal function even if PRL levels remain above the upper limit of normal; in these patients, the biological response rather than the absolute PRL level should be followed with regards to the treatment dose.
Since only 5–10% of microprolactinomas progress to larger tumours, women with microprolactinoma who do not wish to become pregnant may not require therapy with a dopamine agonist. Those who are amenorrhoeic may be treated with oestrogen and should have annual evaluations of serum PRL. An MRI should be repeated if either clinical signs of tumour expansion appear or if the prolactin levels rise significantly.3
Treatment of macroprolactinomas
Dopamine agonist treatment normalizes serum PRL levels and reduces tumour size in most patients with macroprolactinoma. Eighty per cent of prolactinomas treated with dopamine agonists shrink by more than 25% of the original volume, and in almost all patients therapy is associated with a 50% reduction in serum PRL. Tumour shrinkage can often be observed within a week or two after starting therapy, but in some cases may not start for several months. Continued tumour shrinkage may occur for many months or even years. It is useful to repeat the MRI 2–3 months after starting therapy and at longer intervals thereafter. Several studies have demonstrated recovery of impaired anterior pituitary function in association with tumour shrinkage31 and ovulatory menses return in over 90% of premenopausal women. Dopamine agonists usually restore visual function to an extent similar to that produced by surgical decompression of the chiasm in macroprolactinoma patients. Therefore, patients with macroprolactinomas who have visual field defects are no longer considered to be neurosurgical emergencies.
Treatment should aim to normalize PRL levels. However, many consider it best to obtain the lowest possible value of prolactin because this strategy may maximize the chances of tumour reduction and even disappearance.32 Treatment should start at low doses to avoid intolerance and side-effects, with a gradual dose increase. After achieving tumour shrinkage the dopamine agonist dose may be slowly decreased provided PRL levels remain in the normal range. In fact, it has been suggested that fertility is more effectively restored in the presence of normal but not suppressed PRL levels. When the PRL level has been normal for at least 2 years and the size of the tumour decreased by more than 50%, the dose of the dopamine agonist can be gradually decreased, because at this stage low doses are likely to maintain stable PRL levels and tumour size. However, in patients with macroadenomas suspension of therapy may lead to tumour expansion and recurrence of hyperprolactinaemia. For this reason, close follow-up is necessary when the drug is tapered or withdrawn in patients with macroprolactinomas.
Short-term follow-up and resistant prolactinomas
The short-term follow-up of macroadenomas and microadenomas includes periodic evaluation of PRL levels. If a visual defect was present at the time of diagnosis, systematic evaluation by visual perimetry and MRI are mandatory. When a patient does not respond adequately to a dopamine agonist, the prolactinoma is considered resistant. Subsequent treatment options include achieving the maximally tolerated dose, changing to a different dopamine agonist, and considering either pituitary surgery or radiotherapy33 (Fig. 2).
Surgical and radiation treatment of prolactinomas
Trans-sphenoidal surgery
Trans-sphenoidal surgery does not reliably lead to a long-term cure, and recurrence of hyperprolactinaemia is frequent.34–37 The success rate of surgery in microadenomas is about 75%, with higher rates for patients with prolactin levels lower than 200 µg/l (∼4000 mIU/l), small tumours, and amenorrhoea of short duration.38 However, these results come from the most experienced neurosurgeons and outside specialized centres results might be considerably worse. The cure rate for macroprolactinomas is much lower. Those tumours with a cavernous sinus extension essentially cannot be cured by surgery. Recurrence of hyperprolactinaemia after initial normalization occurs in about 20% of patients. Up to 10% of patients may require surgery if they do not respond to dopamine agonists or if visual field deficits do not improve. Other indications for surgery include apoplexy with neurological signs in macroadenomas; cystic macroprolactinomas (which generally do not shrink in response to dopamine agonist treatment) causing neurological symptoms; and intolerance to dopaminergic agonists. In centres with experienced neurosurgeons, the possibility of cure by surgery versus long-term dopamine agonist therapy should be discussed with the patient, and patient preference is also an indication for surgery.
Radiotherapy
External radiation is rarely required to treat prolactinomas and is associated with a significant incidence of major side-effects, including hypopituitarism, damage to the optic nerve, neurological dysfunction and increased risks of stroke and secondary brain tumours. Therefore, radiotherapy is not an acceptable primary therapy for prolactinomas, and is reserved for patients who do not respond to dopamine agonists, those who are not cured by surgery, or for those very rare cases of malignant prolactinoma.
Treatment of malignant prolactinomas
Surgery and radiotherapy are the only treatments available for malignant prolactinomas. Such tumours initially present as resistant prolactinomas or with dissociation between serum PRL levels and tumour mass. However, clinicians should be aware that there is no typical clinical presentation of these tumours except as a failure of dopamine agonist therapy or recurrence after surgery. Usually, pathological information is unremarkable, and the slight increase in the mitotic index is not specific. Extension to noncontiguous areas of the CNS or metastasis to areas outside the CNS may be the first manifestation of a true malignant prolactinoma. Experience managing malignant prolactinomas is very limited. Surgery and radiotherapy are only palliative, and chemotherapy seems to provide little or no benefit. These cancers are uniformly fatal, but fortunately are very rare.
Pregnancy and prolactinoma
Women with prolactinomas who are pregnant or who wish to become pregnant should be guided through the process by an endocrinologist for a number of reasons. During pregnancy, there can be a substantial increase in the volume of the prolactinoma that may in turn compromise visual fields. On the other hand, serum PRL determinations do not reliably reflect an increase in the size of prolactinomas. In addition, dopaminergic drugs cross the placental barrier; hence their effects on the foetus should be carefully considered. There are four main issues with respect to gestation and prolactinomas: hyperprolactinaemia and fertility, safety of dopamine agonists, tumour growth, and lactation.
Hyperprolactinaemia and fertility
When starting dopaminergic treatment, women must be warned that restoration of ovulation and fertility may be immediate (even before their first normal menstruation). For that reason, when starting dopamine agonist treatment, mechanical contraception should be advised, and menses may serve as a guide. When a female patient with a macroprolactinoma wishes to become pregnant, it is necessary to plan conception to occur after serum PRL is normalized and the tumour volume significantly reduced in order to avoid or reduce the risk of compression of the optic chiasm during pregnancy. Trans-sphenoidal surgery is an option in an infertile patient with a prolactinoma who cannot tolerate dopaminergic drugs or is resistant to them.
Safety of dopamine agonists
There is considerable worldwide experience with patients who become pregnant while taking bromocriptine, and also with the use of this drug for long periods during pregnancy. The incidence of abortions, ectopic pregnancies or congenital malformations is no higher in infants born to mothers who conceived while taking bromocriptine than that in the general population.39,40 Similar results have been reported with women who have taken cabergoline before and during pregnancy;41–47 however, the number of pregnancies studied using cabergoline is smaller than for bromocriptine. In experimental models cabergoline administration during pregnancy has not been found to induce teratogenicity.48 The experience with pergolide and quinagolide in preparation for pregnancy is much more limited; for that reason these two drugs should not be used in this setting.
Although the use of bromocriptine and cabergoline is not associated with increased problems, it is best to limit the exposure of the embryo to such drugs as much as possible. Teratogenicity from any drug or cause most often occurs during the first trimester of pregnancy. The first trimester coincides with the period of lowest growth of a macroadenoma in pregnant women who have stopped therapy. Therefore, it is recommended that bromocriptine or cabergoline be stopped once the first menstrual period has been missed and a positive pregnancy test is obtained. Cabergoline in particular has very prolonged action, and PRL levels may be suppressed for up to 120 days after withdrawal.41
Tumour growth
For women with microprolactinomas the risk of clinically relevant tumour expansion is less than 2% during pregnancy. Therefore, dopamine agonists can be safely stopped in such patients as soon as pregnancy has been confirmed. The patients should be advised to report for urgent assessment in the event of a severe headache or visual disturbance. Serial PRL determinations are not necessary. In women with macroadenomas, symptomatic tumour expansion occurs in 20–30% of women. Options for such women include stopping the dopamine agonist when pregnancy is confirmed with close surveillance thereafter, or continuing the dopamine agonist through the pregnancy. If visual field defects or progressive headaches develop, an MRI without gadolinium (not a CT) should be performed to assess changes in tumour size, and a dopamine agonist should be restarted if the tumour has grown significantly.49 Debulking surgery before pregnancy in women with macroprolactinomas to reduce the likelihood of major tumour expansion is a less preferable option, since medical therapy during pregnancy is probably less harmful than surgery. If the enlarged tumour does not respond to reinstitution of dopamine agonist therapy, alternatives include delivery if the pregnancy is far enough advanced, or trans-sphenoidal surgery.44,45
Lactation
Women wishing to breast-feed their infants should not be given dopamine agonists because the resulting decrease in serum PRL levels will impair lactation. There are no data to suggest that breast-feeding leads to an increase in tumour size.
Long-term follow-up
The minimal length of dopamine agonist therapy should be 1 year. Importantly, some patients may remain in long-term remission after a period of several years of dopamine agonist treatment. There are no signs to predict whether drug discontinuation will be successful, but a recent report indicates that dopamine agonists can be safely withdrawn in patients with long-term normalization of prolactin levels and no evidence of tumour on MRI.50–52 If a patient has normal PRL levels after therapy with dopamine agonists for at least 3 years and the tumour volume is markedly reduced, a trial of tapering and discontinuation of these drugs may be initiated. Such patients need to be carefully followed to detect recurrence of hyperprolactinaemia and tumour enlargement so that treatment can be promptly resumed.
Acknowledgements
The highly effective work of Dr Tatiana Mancini is gratefully acknowledged.




