Causes of status epilepticus
Summary
Status epilepticus (SE) is the most extreme form of epilepsy. It describes a prolonged seizure that may occur in patients with previous epilepsy or in acute disorders of the central nervous system. It is one of the most common neurologic emergencies, with an incidence of up to 41 per 100,000 per year and an estimated mortality is 20%. The three major determinants of prognosis are the duration of SE, patient age, and the underlying cause. Common and easily recognized causes of SE include cerebrovascular disorders, brain trauma, infections, and low antiepileptic drug levels in patients with epilepsy. Less common causes present a clinical and diagnostic challenge, but are major determinants of prognosis. Among them, inflammatory causes and inborn errors of metabolism have gained wide interest; recent insights into these causes have contributed to a better understanding of the pathophysiology of SE and its appropriate treatment. This review focuses on the different etiologies of SE and emphasizes the importance of prompt recognition and treatment of the underlying causes.
Status epilepticus (SE) is a term used to describe a prolonged and self-sustaining seizure that may have overt, subtle, or almost no behavioral manifestations. It may be regarded as the most extreme form of epilepsy, or as an expression of an acute and often life-threatening brain disorder, such as stroke, encephalitis, or trauma. Mortality associated with SE is up to 20% (Shorvon, 1994; Logroscino et al., 1997, 2005). Less than 50% of people in SE have had previous seizures or epilepsy (DeLorenzo et al., 1996; Hesdorffer et al., 1998; Coeytaux et al., 2000; Knake et al., 2001; Vignatelli et al., 2003). SE is one of the most common neurologic emergency, with an overall annual incidence of 10–41 per 100,000 (DeLorenzo et al., 1996; Hesdorffer et al., 1998; Coeytaux et al., 2000; Knake et al., 2001; Vignatelli et al., 2003). Up to 287,000 patients per year are affected in Europe. Not all forms of status are life-threatening and, given the variety of its clinical presentations, the management must be tailored according to the type of SE and the underlying cause. Three major factors determine an increased risk of mortality and morbidity associated with SE: (1) certain etiologies, (2) age >60 years, and (3) long duration of SE (Towne et al., 1994; DeLorenzo et al., 1996; Wu et al., 2002; Rossetti et al., 2006). This review focuses on the various causes of SE, since etiology is increasingly recognized as one of the most important factors for prognosis and outcome. Therefore, identifying and treating the underlying cause of SE is at least as important as prompt and effective treatment with early termination of seizures.
Definition and Classification of Status Epilepticus
Status epilepticus (SE) has been recognized for centuries (Shorvon, 1994; Wolf et al., 2009). Henri Gastaut (1970) recognized SE as a prolonged seizure with as many forms as there were types of epileptic seizures. Therefore, SE classification mirrored exactly the seizure classification. In the International League against Epilepsy (ILAE) 1981 Classification, SE was defined as “a seizure that persists for a sufficient length of time, or repeated frequently enough that recovery between attacks do [does] not occur” (Commission on Classification, ILAE, 1981). Generalized tonic–clonic seizures usually do not last longer than 2–3 min (Theodore et al., 1994); the risk of a seizure becoming self-sustaining increases as the duration reaches 5 min or more (Lowenstein et al., 1999). For the purpose of this review, we use a clinical classification along two taxonomic criteria: the presence (or absence) of motor symptoms and the impairment (or retention) of consciousness. Therefore, one can distinguish (A) SE types with prominent motor symptoms, such as convulsive SE, and (B) SE types without prominent motor symptoms, summarized as nonconvulsive SE (NCSE). NCSE can occur with or without coma; this has important etiologic implications and determines its treatment. A third category (C) comprises the boundary syndromes, including epileptic encephalopathies and acute forms of coma with status-like electroencephalography (EEG) patterns. Table 1 briefly outlines this tentative classification. For a more detailed discussion of SE classification, refer to: (Shorvon, 1994; Walker et al., 2005; Bauer & Trinka, 2006, 2009; Berg et al., 2010). However, the definitions and classifications are in flux and an ILAE Task force is currently developing a new draft classification of SE, following the concepts of the ILAE’s new classification proposal of seizures and syndromes (Berg et al., 2010).
| With prominent motor symptoms |
| Convulsive SE (syn.: tonic–clonic SE) |
| Myoclonic SE (prominent epileptic myoclonic jerks) |
| Focal motor (including EPC) |
| Tonic SE |
| Hyperkinetic SE |
| Without prominent motor symptoms (i.e., NCSE) |
| NCSE with coma |
| NCSE without coma |
| Generalized |
| Focal |
| Boundary syndromes |
| Epileptic encephalopathy |
| Acute forms of coma with status-like EEG pattern |
| Epileptic behavioral disturbance and psychosis |
| Confusional states, or delirium with epileptiform EEG changes |
Causes of Convulsive Status Epilepticus in Adults
Most population-based studies have used a traditional 30-min duration of SE, and so the numbers given are the lowest estimates. Using the 5-min definition, determining the time from onset to starting emergency treatment, the incidence in clinical practice is much higher than in the epidemiologic studies. Convulsive SE comprises 37–70% of all forms of status, and its annual incidence is up to 40 per 100,000 (Waterhouse, 2008). In adults with preexisting epilepsy, the most common etiologies are low antiepileptic drug (AED) levels (accounting for at least one fourth of SE [Fig. 1]), remote symptomatic etiologies, and stroke (DeLorenzo et al., 1995, 1996). This subgroup with epilepsy and low AED levels has a good prognosis, with a low mortality of 4.0–8.6% (Towne et al., 1994; DeLorenzo et al., 1995). Overall, acute symptomatic causes are the most common etiology, accounting for 48–63% of all SE cases (Hesdorffer et al., 1998; Coeytaux et al., 2000; Knake et al., 2001). Stroke is the leading cause among the acute symptomatic cases, accounting for 14–22% of SE in adults (DeLorenzo et al., 1995; Knake et al., 2001). In older adults, remote stroke is a major cause. Knake et al. (2001) found that remote stroke caused 36% of SE in patients older than 56 years. In the Richmond Virginia Status Epilepticus Study, 41% of adults and 61% of the elderly had acute or remote ischemic and hemorrhagic strokes as cause of status (DeLorenzo et al., 1995).
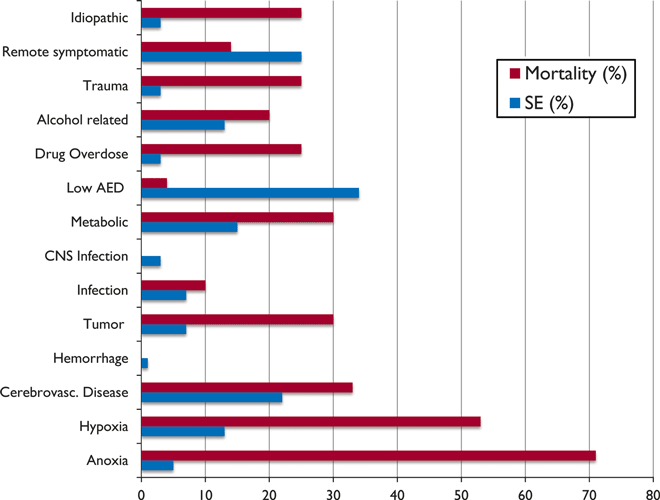
Etiology of status epilepticus in adults, with associated mortality for each category. Based on data from DeLorenzo et al., 1995. AED, antiepileptic drugs; CNS, central nervous system.
In the context of epilepsy, SE may develop in those with a previous diagnosis of epilepsy or de novo, as its initial manifestation. Approximately 15% of patients with epilepsy have had at least one episode of status during their lifetime. Most often, the SE is due to the epilepsy itself, triggered by medication nonadherence, resulting in subtherapeutic AED levels (Aminoff & Simon, 1980) or by inappropriate drug treatment (Thomas et al., 2006a,b). The clinical features of SE in these patients depend on the underlying epilepsy syndrome. In the context of idiopathic generalized epilepsy, status is most often nonconvulsive (Shorvon & Walker, 2005); in the context of juvenile myoclonic epilepsy it may be myoclonic (Thomas et al., 2006a,b; Larch et al., 2009). Myoclonic status may also develop in progressive myoclonic epilepsy, Lennox-Gastaut syndrome, or epilepsy with myoclonic absences. However, the precise incidence of convulsive status or myoclonic status in these syndromes is not known.
Approximately 12% of patients who eventually develop epilepsy have presented with SE as their first clinical manifestation (Hauser, 1990; Hesdorffer et al., 1998). In these patients, SE may be an intrinsic manifestation of disease, sometimes with recurrent episodes, or the epilepsy may be the consequence of a prolonged SE, with neuronal death and alteration of networks causing recurrent seizures after the initial event. During a 10-year follow-up, epilepsy developed in 42% of patients who had acute symptomatic SE and in 14% of patients who had acute symptomatic seizures (Hesdorffer et al., 1998). The development of subsequent epilepsy is more likely if the status is refractory to treatment (Holtkamp et al., 2005), supporting the hypothesis that SE contributes to epileptogenesis by enhancing hyperexcitable networks.
Nonconvulsive Status Epilepticus
NCSE may be defined as “an enduring epileptic condition with reduced or altered consciousness, behavioral and vegetative abnormalities, or merely subjective symptoms without major convulsive movements” (Drislane, 2000). This umbrella term includes a wide spectrum of disorders, ranging from benign conditions, such as absence status in idiopathic generalized epilepsy, to severe life-threatening conditions, such as subtle SE or coma with generalized epileptiform discharges (coma-GEDs). Therefore, it is important to subdivide this category according to the degree of unresponsiveness or to the depth of coma (Fig. 2). Consciousness, which notoriously resists definition, becomes a taxonomic criterion for subdividing NCSE (Bauer & Trinka, 2009). Consciousness itself can be categorized into quantitative and qualitative consciousness. The quantitative element depends upon the patient’s level of consciousness and arousability, which in turn depends upon the integrity of the ponto-mesodiencephalic reticular pathways and the thalamocortical projections. Qualitative consciousness, on the other hand, depends upon the content of consciousness, experience, emotions, and sensations, known only to patients themselves. It reflects the inner monologue and it is essential for any meaningful interaction with the environment. Qualitative consciousness is associated with awareness, enabling the patient either to focus on and interact with the environment or to engage in an inner monologue. Table 2 describes the different types of NCSE.
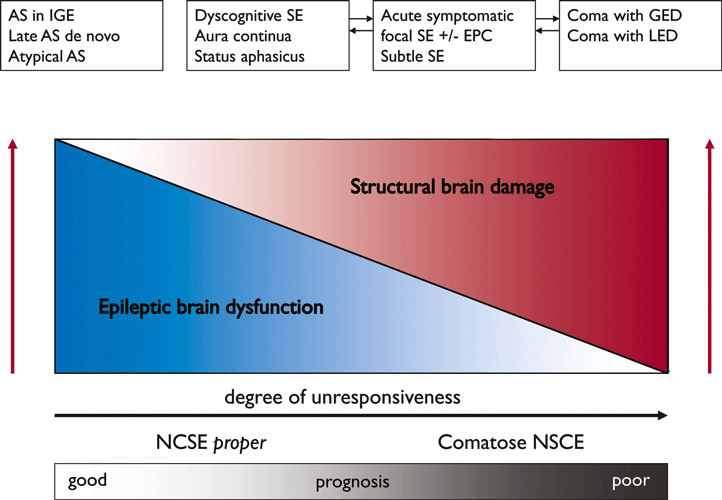
Relationship between depth and coma (x-axis), prognosis (x-axis), degree of structural brain damage (red y-axis), and epileptic brain dysfunction (blue y-axis) due to status epilepticus. Clinical entities depicted in the upper part of the graph are arranged along the x-axis without distinct positions, in recognition that large border zones and overlaps between the conditions may exist. With permission from Bauer & Trinka, 2009. AS, absence status epilepticus; EPC, epilepsia partialis continua; GEDs, generalized epileptiform discharges; IGE, idiopathic generalized epilepsy; LEDs, lateralized epileptiform discharges; NCSE, nonconvulsive status epilepticus.
| NCSE with coma |
| NCSE without coma |
| Generalized |
| Typical absence status |
| Atypical absence status |
| Myoclonic absence status |
| Focal |
| Aura continua |
| With vegetative symptoms |
| With sensory symptoms |
| With visual symptoms |
| With olfactory symptoms |
| With gustatory symptoms |
| With emotional symptoms |
| Aphasic SE |
| SE with dyscognitive symptoms |
The incidence of NCSE, based on epidemiologic studies, ranges from 40.0 to 66.9% (DeLorenzo et al., 1995; Hesdorffer et al., 1998; Coeytaux et al., 2000; Knake et al., 2001; Vignatelli et al., 2003). Like convulsive status, NCSE may occur in acute central nervous system (CNS) disorders or may be a part of certain electroclinical syndromes. We do not know the precise incidence of NCSE in the epilepsy population. In our own audit (1975 to 2003) at the University Hospital Innsbruck, 220 patients with epilepsy (140 women, median age 45 years [range 2–89]) had at least one episode of NCSE either during their disease or at their initial presentation (Bauer G, Trinka E, unpublished data). The cause of NCSE was remote symptomatic in 47%, idiopathic in 45%, and remained unknown (cryptogenic) in 48%. Forty-six percent had focal SE, 21% had a generalized NCSE in the context of idiopathic (or genetic) generalized epilepsies, and 22% had atypical absence status in the context of Lennox-Gastaut syndrome. Table 3 details their different types of status epilepticus.
| n | % | |
|---|---|---|
| Generalized (106 = 48.2%) | ||
| Typical absence SE | 57 | 25.9 |
| Atypical absence SE | 49 | 22.3 |
| Localized/lateralized (100 = 45.5%) | ||
| Focal simple (58 = 26.4%) | ||
| Aura continua | 11 | 5.0 |
| Vegetative | 8 | 3.6 |
| Aphasic | 27 | 12.3 |
| Pure frontal | 9 | 4.1 |
| Psychosis | 3 | 1.4 |
| Focal complex (42 = 19.1%) | ||
| Continuous | 30 | 13.7 |
| Discontinuous | 12 | 5.5 |
| Unclassified | 14 | 6.3 |
| Total | 220 | 100.1 |
Causes of Absence Status Epilepticus
Absence status (AS) is best described as “a confusional state of variable intensity, ranging from simple cognitive slowing to catatonic stupor, lasting for hours to days or weeks” (Andermann & Robb, 1972). The EEG shows bilateral rhythmic, synchronous, and mostly symmetric paroxysmal activity, which can be continuous or discontinuous (Bauer & Trinka, 2010; Fig. 3). It is important to recognize that AS is a heterogeneous condition, which may occur in patients with preexisting idiopathic generalized epilepsy (“typical” AS) or during the course of chronic symptomatic generalized epilepsy, such as Lennox-Gastaut syndrome (“atypical” AS). Of interest, some patients develop AS later in life, occasionally de novo (Andermann & Robb, 1972; Thomas et al., 1992), or it may occur as a late exacerbation of an idiopathic generalized epilepsy syndrome (Bauer et al., 2007). There may also be a fourth group of AS, comprising “absences” with very clear focal characteristics. This group may be considered as a transitional form between AS and dyscognitive focal SE of frontal origin (Bauer et al., 2006; Thomas, 2011).
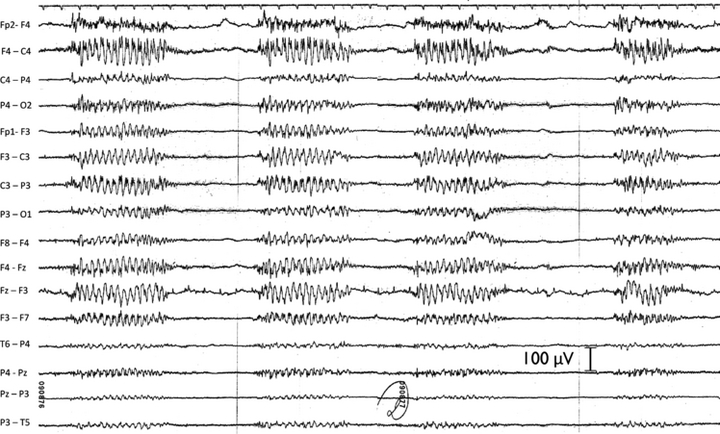
Typical absence status epilepticus in an older patient (V.J., female, 74 years. tc 0.3 F30). This patient had scattered generalized tonic–clonic seizures since age of 54 years. Her EEG showed periodically repeated generalized 3/s spike and wave, with no major diffuse slow activity between periods (note the reduced time calibration). EEG kindly provided by Prof. Dr. G. Bauer, Innsbruck. The patient displayed discontinuous psychic functions, and was amnesic for the abnormal condition. Recovery was immediate after intravenous diazepam.
Many cases of AS have nonspecific precipitating factors. The most important are antiepileptic drug (AED) withdrawal or impaired adherence, alcohol, sleep deprivation, and sleep–wake cycle disturbance. Other nonspecific factors include stress, fatigue, fever, mild head trauma, or metabolic derangement after surgery (Thomas & Zifkin, 2008; Thomas & Gelisse, 2009).
Several idiopathic generalized epilepsy syndromes may be aggravated by inappropriate AEDs: carbamazepine, phenytoin, tiagabine, or other γ-aminobutyric acid (GABA)ergic medications (Snead & Hosey, 1985; Knake et al., 1999; Thomas et al., 2006a,b; Trinka et al., 2002). Thomas et al. reported 14 patients with idiopathic generalized epilepsy treated with either carbamazepine alone or with other potentially aggravating drugs, for example, phenytoin, vigabatrin, or gabapentin. Ten of these cases developed AS; in half, the AS was atypical. All their patients had a clear seizure aggravation, with development of new seizure types before AS developed (Thomas et al., 2006a,b). The prognosis was invariably good with full seizure control in all patients after switching to appropriate drugs.
In late-onset AS, other drug-related factors play an important clinical role (Thomas et al., 1992; Thomas & Andermann, 1994). Most importantly, psychotropic medication—or its withdrawal—may provoke late-onset AS (Fernandez-Torre, 2001). In addition, many other drugs, for example, theophylline, baclofen, metformin, and cimetidine, may exacerbate absences later in life (for a review see Thomas & Snead, 2007).
There are metabolic and toxic factors in many patients, although the precise incidence is not known. Examples include hyponatremia, hypoglycemia decompensated chronic renal failure, hepatic failure, and hypocalcemia (for review see Thomas & Snead, 2007). There have been several cases of AS following the use of contrast-enhancing products during myelography or carotid angiography (Pritchard & O’Neil, 1984; Vollmer et al., 1985; Coeytaux et al., 2000). Some cases of transient global amnesia after angiography may in fact also be epileptic in nature (Bauer et al., 2005).
AS probably occurs most frequently in the context of electroclinical syndromes. Aside from childhood absence epilepsy, juvenile absence epilepsy, and juvenile myoclonic epilepsy, there are other syndromes associated with AS as a key clinical feature: AS may occur in eyelid myoclonia with absences (Jeavons syndrome) (Yang et al., 2008), idiopathic generalized epilepsy with phantom absences (Panayiotopoulos et al., 2001), perioral myoclonias with absences (Agathonikou et al., 1998), or absences status epilepsy (Genton et al., 2008). The prognosis in these syndromes (not yet fully accepted in the ILAE classification) is generally good, and patients respond well to appropriate AEDs—valproate in most cases. However, this is not true of AS associated with ring chromosome 20, where prolonged confusional states are notoriously resistant to AEDs and there have been lethal cases (Jacobs et al., 2008). The EEG shows bilateral high-voltage slow waves, sometimes with intermingled spikes and frontal accentuation. There is no typical clinical picture, except for mild cognitive impairment (Fig. 4).
Clinical course of convulsive status epilepticus.
Causes of Focal Nonconvulsive Status Epilepticus
Focal NCSE (as with AS) encompasses a wide range of clinical symptoms. Previously the term “complex focal or complex partial” SE was used, which may be replaced in future by “dyscognitive SE.” These forms usually have structural abnormalities and clinical focal signs that must be identified to guide appropriate drug treatment (Fig. 2).
Most of the published literature concerns patients with temporal or extratemporal lobe epilepsy of remote symptomatic cause, whose NCSE is either as the presenting symptom or develops during the course of the disease (Tomson et al., 1992, Kaplan et al., 1996; Scholtes et al., 1996). As with AS, there are often nonspecific risk factors, but more often status occurs in these patients without specific provoking factors. In a review of 70 patients with focal NCSE of frontal origin, more than one fourth had no history of epilepsy. Forty-five percent had a focal frontal lesion. The etiology was most often a brain tumor (benign or malignant), or a posttraumatic or a postsurgical lesion (Thomas & Zifkin, 2008).
Rare causes of focal NCSE include intravenous contrast media, or drugs such as ciprofloxacin, lithium intoxication, theophylline, vigabatrin, tiagabine, or crack cocaine (Thomas et al., 2006b). Mesial temporal focal NCSE can also be the presenting symptom of the increasingly recognized nonparaneoplastic limbic encephalitis, related to voltage-dependent potassium channels (VGKC/LGI1) (Irani et al., 2010), NMDA receptors (Vincent & Bien, 2008), or antibodies against glutamic acid decarboxylase (Malter et al., 2010). Some of them lead to a specific seizure type (e.g., faciobrachial dystonic seizures associated with anti-VGKC/LGI1 antibodies), preceding the onset of the limbic encephalitis (Irani et al., 2011a,b). It is important to note that paraneoplastic limbic encephalitis may also present with focal NCSE (Dalmau et al., 2008), indicating the need for a comprehensive search and rigorous treatment of any underlying primary neoplasm. We do not yet know the full spectrum of this new group of immune-mediated encephalopathies (Table 4).
| Paraneoplastic encephalitis |
| Hashimoto encephalopathy |
| Anti-NMDA-receptor encephalitis |
| Anti-VGKC-receptor encephalitis |
| Rasmussen encephalitis |
| Cerebral lupus |
| Adult-onset Still disease |
| Anti-GAD antibody associated encephalitis |
| Goodpasture syndrome |
| Thrombotic thrombocytopenic purpura |
| Antibody-negative limbic encephalitis |
- NMDA, N-methyl-d-aspartate; GAD, glutamate acid decarboxylase.
The outcome for focal NCSE is less favorable than that for AS. Long-lasting focal status, especially of temporal lobe origin, may cause brain edema identifiable on magnetic resonance imaging (MRI) (Bauer et al., 2006). These patients may also have severe and prolonged amnesia (Engel et al., 1978; Treiman et al., 1981). However, many patients presenting with focal NCSE have acute or remote symptomatic epileptogenic lesions, making it difficult to disentangle the dysfunction due to the epileptic activity from the effect of the structural lesion (Hilkens & De Weerd, 1995; Kaplan, 1996; Trinka et al., 2002). However, focal NCSE occurring with an acute lesion most likely contributes substantially to the associated neurologic dysfunction (Hilkens & De Weerd, 1995; Bauer & Trinka, 2010). Therefore, patients with focal NCSE need prompt and vigorous treatment, tailored to the underlying cause.
Causes of Comatose Nonconvulsive Status Epilepticus
Comatose forms of NCSE need further attention. It is important from the clinical standpoint (1) to clarify whether the coma is caused by the epileptic seizure or status, or by the brain disorder itself, (2) to assess the contribution of epileptic activity to the depth of coma, (3) to consider whether treatment improves prognosis in these patients or not, and (4) to implement an appropriate treatment in these critically ill patients. Unlike in other forms of NCSE, the cause of the comatose patient’s SE can sometimes only be identified from the history, the temporal pattern of coma, and the neurologic signs. The epileptic etiology is most often confirmed only by the presence of continuous or discontinuous epileptiform discharges on the EEG (Lowenstein & Aminoff, 1992; Jordan, 1999; Brenner, 2005). A frequently used, but not universally accepted, classification of patients in comatose status uses the dichotomy of generalized versus focal or lateralized EEG discharges (Brenner, 2004). The EEG is absolutely necessary to make this distinction. In this review we used the terms “coma with generalized epileptiform discharges” (or coma-GED) and “coma with lateralized epileptiform discharges” (or coma-LED) (Bauer & Trinka, 2009). The etiology in these patients is almost always acute symptomatic, encompassing a wide variety of systemic diseases or CNS disorders. Table 5 shows the etiologic factors and EEG patterns found in coma-GED and coma-LED. Unlike in AS and focal NCSE, these patients are often resistant to treatment, and the prognosis depends entirely on the course of the underlying disorder. Advanced convulsive SE becomes oligosymptomatic with fewer motor symptoms; deep coma is the leading symptom, hence the term “subtle status” (Treiman et al., 1984). The causes are identical to convulsive SE.
| Etiology | EEG pattern | |
|---|---|---|
| coma-GED | Diffuse primary or secondary brain disturbances (anoxic, toxic, metabolic, infectious, degenerative) Space-occupying lesions with brainstem compression (direct or due to tentorial herniation)aKnown epilepsies? | Continuous generalized spiking Periodic spiking Burst suppression pattern in different variations Other generalized periodic abnormalities Bilateral triphasic waves |
| coma LED | Focal brain lesions (in most cases acutely acquired) In rare cases diffuse abnormalities (aminophylline, intoxiation, some forms of diabetic coma) Known epilepsies? | Continuous focal spiking PLEDs and Bi-PLEDS Unilateral burst suppression pattern unilateral triphasic waves |
- Bi-PLED, bilateral periodic epileptiform discharges; GEDs, generalized epileptiform discharges; LED, lateralized epileptiform discharges; PLEDs, periodic epileptiform discharges.
- a Might also present as coma-LED.
Causes of Epilepsia Partialis Continua
Epilepsia partialis continua (EPC) is a special type of focal status epilepticus (Table 1), first described by Kojewnikov in 1894; he considered it as a “peculiar form of cortical epilepsy.” EPC is characterized by “spontaneous regular or irregular clonic muscle twitching of cerebral cortical origin, sometime aggravated by action or sensory stimuli, confined to one part of the body, and continuing for a period hours days, or weeks” (Obeso et al., 1985). EPC, also called Kojewnikov syndrome, occurred in patients with Russian spring–summer tick-borne encephalitis; EPC typically develops 2–3 weeks after the end of the acute illness (Zemskaya et al., 1991). EPC was later recognized to occur in several neurologic disorders, with age of onset ranging from infancy to adulthood, and even in the elderly. The prevalence, based on the EPC case registry of the British Neurological Surveillance Unit, is <1 per million (Cockerell et al., 1996).
The reported causes of EPC are extremely diverse, and include metabolic disorders (nonketotic hyperglycemia or mitochondrial encephalopathy), cerebrovascular disorders, inflammation (especially Creutzfeldt–Jakob disease, progressive multifocal leukoencephalopathy from HIV infection, multiple sclerosis, neurocysticercosis and others), neoplasms (astrocytoma, hemangioma, lymphoma and metastasis), and cortical dysplasia (focal cortical dysplasia and hemimegalencephaly) (Oguni et al., 1991; Veggiotti et al., 1995; Lee et al., 2000; Placidi et al., 2001; Pandian et al., 2002; Huang et al., 2005; Wieser & Chauvel, 2005; Aydin-Ozdemir et al., 2006; Kinirons et al., 2006; Sinha & Satischandra, 2007; Bien & Elger, 2008; Yeh & Wu, 2008; Mameniskiene et al., 2011). The most common etiologies are cerebrovascular disorders (24–28%), inflammatory causes (15–19%), neoplasms (5–16%), and metabolic disorders (6–14%). Despite full neurologic work-up, 19–28% of EPC cases have no identifiable cause (Thomas et al., 1977; Cockerell et al., 1996; Sinha & Satischandra, 2007). Table 6 gives an overview of the causes of EPC.
| Age period | EPC type 1 (static cause) | Diagnostic test | EPC type 2 (progressive cause) | Diagnostic test |
|---|---|---|---|---|
| Infancy | Hemimegalencephaly | Mitochondrial disease (Alper disease) | Serum and cerebrospinal fluid lactate, muscle biopsy biopsy, mitochondrial DNA (mutation of POLG1) | |
| Childhood | Focal cortical dysplasia Sturge-Weber syndrome focal cortical dysplasia | Rasmussen syndrome | Cerebrospinal fluid oligoclonal banding, immunoglobulin G index | |
| Tuberous sclerosis | Repeated skin examination | Mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) | Serum and cerebrospinal fluid lactate, muscle biopsy, mitochondrial DNA | |
| Neurocysticerosis | Immunoelectrotransfer blot assay | Delayed type of measles encephalitis (complication of measles in immunocompromised children) | Immunosuppresive treatment, contact with measles | |
| (Tick-borne) encephalitis | Cerebrospinal fluid study serologic test for virus | |||
| Gliomatosis cerebri Other foreign tissue lesions Nonketotic (ketotic) hyperglycemia | Serum glucose, urinary ketones | |||
| Adults | Cerebrovascular disorders (stroke; intracranial bleeding, cerebral venous thrombosis, vasculitis) | Adult-onset Rasmussen’s syndrome | Cerebrospinal fluid oligoclonal banding, immunoglobulin G index | |
| Nonketotic (ketotic) hyperglycemia | Serum glucose | Creutzfeldt-Jakob disease | 14-3-3 protein in cerebrospinal fiuid | |
| Focal cortical dysplasia | Myoclonus epilepsy with ragged red fibers (MERRFs) | Serum and cerebrospinal fluid lactate, muscle biopsy, mitochondrial DNA | ||
| Paraneoplastic limbic encephalopathy | Cerebrospinal fluid study, chest computed tomography, anti-Hu test | Kuf’s disease | Skin or rectal mucosal biopsy | |
| Neoplasms Tuberculous meningitis (tuberculoma) (Tick-borne) encephalitis | Cerebrospinal fluid study, chest XR, tuberculin skin test Cerebrospinal fluid study, serologic test for virus | |||
| Autoimmune thyroid encephalopathy | Thyroid function tests, antithyroglobulin antibody, antimicrosomal antibody | |||
| Behcet disease | Neuroimaging, recurrent oral and genital ulceration, skin lesions, HLA-B5 positivity | |||
| Sjögren syndrome | Hypergammaglobulinemia positive antinuclear antibody, anti-SSA, SSB, rheumatoid factor | |||
| Multiple sclerosis | Cerebrospinal fluid oligoclonal banding | |||
| HIV encephalopathy | Immunoglobulin G index Serologic test for HIV |
Bancaud divided EPC into two types: type 1, caused by focal static lesions of the sensorimotor cortex, and type 2, caused by progressive cerebral lesions with neurologic and intellectual deterioration (Bancaud, 1992). Type 2 most often reflects mitochondrial diseases and inflammatory causes, such as Rasmussen syndrome or Creutzfeldt–Jakob disease.
Uncommon Causes of Status Epilepticus
The uncommon causes of SE deserve consideration. In many cases of drug-resistant SE, the underlying disorder remains untreated, because rare causes may be overlooked. We do not know the frequency of these rare causes, and the available literature is mostly confined to single case reports or small case series. This overview is based on a systematic search of all available English literature between 1990 and 2008 (Tan et al., 2010). The authors identified 181 causes of SE after reviewing 513 articles. The causes fell into five categorical groups:
| Alpers disease |
| Occipital lobe epilepsy/mitochondrial spinocerebellar ataxia and epilepsy (MSCAE) |
| Mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) |
| Leigh syndrome |
| Myoclonic encephalopathy with ragged red fibers (MERRF) |
| Neuropathy, ataxia, and retinitis pigmentosa (NARP) |
| Atypical bacterial infections | Viral infections | Prion disease | Other infections |
|---|---|---|---|
| Bartonella/cat-scratch disease | HIV and HIV-related infections | Creutzfeldt-Jakob disease | Paracoccidioidomycosis |
| Coxiella burnett (Q fever) | West Nile encephalitis | Paragonimiasis | |
| Neurosyphilis | JC virus (progressive multifocal leukoencephalopathy) | Mucormycosis | |
| Scrub typhus | Parvovirus B19 | ||
| Shigellosis | Varicella encephalitis | ||
| Mycoplasma pneumonia | Subacute sclerosing panencephalitis | ||
| Chlamydophilapsittaci | Measles encephalitis Rubella encephalitis Rous sarcoma virus (RSV) associated SE Polioencephalomyelitis St. Louis encephalitis |
| Chromosomal aberrations | Inborn errors of metabolism | Malformations of cortical development | Neurocutaneous syndromes | Others |
|---|---|---|---|---|
| Ring chromosome 20 | Porphyria | Focal cortical dysplasias | Sturge-Weber syndrome | Rett’s syndrome |
| Angelman syndrome | Menke’s disease | Hemimegalencephaly | Tuberous sclerosis | Dravet syndrome and SCN1A gene mutation spectrum |
| Wolf-Hirschhorn syndrome | Wilson’s disease | Polymicrogyria | Migrating partial seizures in infancy | |
| Fragile X syndrome | Alexander’s disease | Heterotopias | Pyeridoxine dependency | |
| X-linked mental retardation syndrome | Gobalamin C/D deficiency | Schizencephaly | Familial hemiplegic migraine | |
| Ring chromosome 17 | Ornithine transcarbamylase (OTC) deficiency Hyperprolinemia Maple-syrup urine disease 3-Methylcrotonyl CoA carboxylase deficiency Lysinuric protein intolerance Hydroxyglutaric aciduria Metachromatic leukodystrophy Kuf’s disease Late infantile ceroid lipofuscinosis Beta-ureidopropionase deficiency | Lafora’s disease Dentato-rubro-pallido-luysian atrophy Infantile-onset spinocerebellar ataxia Wrinkly-skin syndrome Neurocutaneous melanomatosis Neuroserpin mutation Wolfram syndrome Autosomal recessive hyperekplexia Cockayne syndrome Cerebral autosomal dominant arterio-pathy with subcortical infarcts and leuko encephalopathy (CADASIL) | ||
| 3-Hydroxyaxyl CoA dehydrogenase deficiency Carnitine palmitoyltransferase Succinic semildehyde dehydrogenase deficiency | Jeavons syndrome Robinow syndrome LYK5 mutation MECP2 mutation | |||
| Malignant hyperpyrexia |
Table 10 lists other uncommon causes of SE. It cannot be overemphasized that the knowledge of the range of conditions is important to clinical practice; the underlying disorder must be treated to achieve full seizure control. The tables are derived from the article by Tan et al. (2010) and reflect the current state of knowledge on these causes of SE.
| Iatrogenic | Other medical conditions |
|---|---|
| Electroconvulsive therapy Temporal lobectomy and other neurosurgery Insertion of intracranial electrode Ventriculoperitoneal shunt Blood transfusion Carotid angioplasty and stenting | Multiple sclerosis Hypertension-induced posterior reversible encephalopathy syndrome Panayiotopoulos syndrome Thyroid disease Pyridoxine-dependent seizure Neuroleptic malignant syndrome Ulcerative colitis Behcet syndrome Celiac disease Cobalamin deficiency Folinic acid responsive seizures Renal artery stenosis Pituitary apoplexy Renal artery dissection Hypomelanosis of Ito Cerebral palsy Hemophagocytic lymphohistiocytosis Anhidrotic ectodermal dysplasia Methemoglobinemia |
Therapeutic Considerations
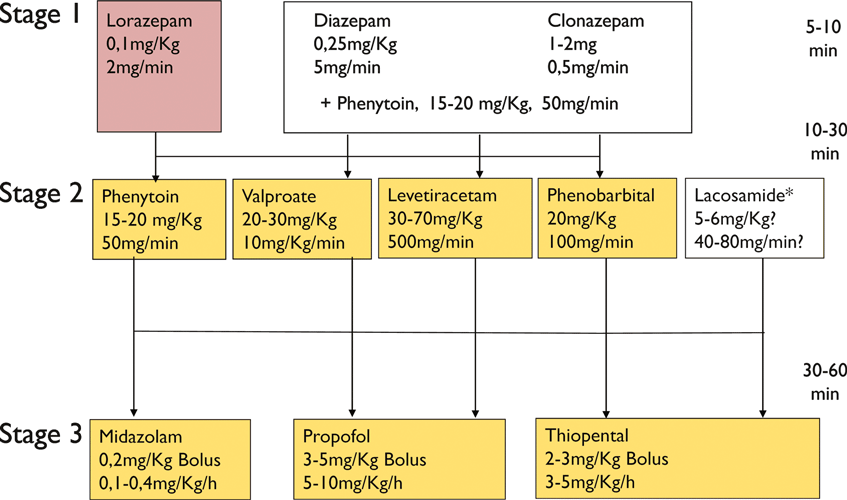
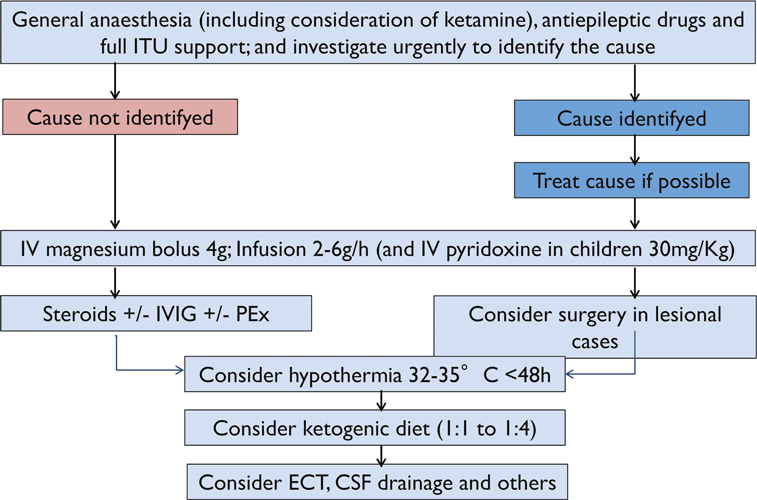
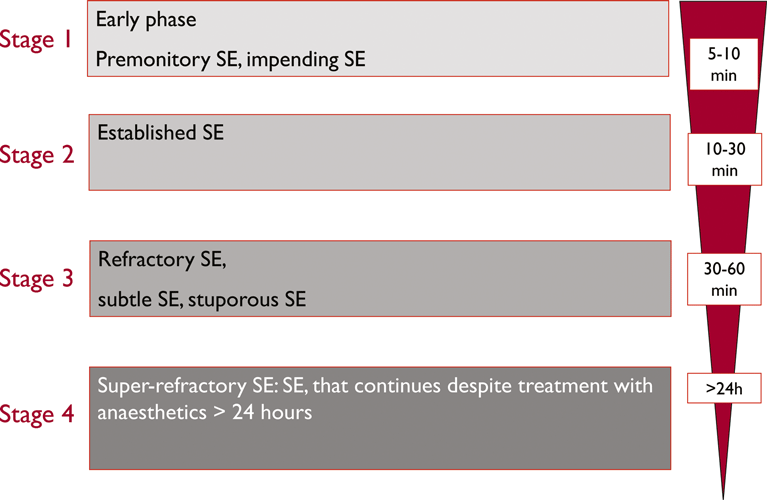
Given the wide variety of clinical presentations of SE, ranging from life-threatening conditions to seemingly harmless ones, it is important to tailor the treatment to the type of status and to the underlying disorder. The clinical presentation of SE determines the aggressiveness treatment. All treatment concepts on convulsive SE are based on a staged approach (Fig. 4). In the early phase of convulsive SE, large randomized controlled trials support the use of intravenous benzodiazepines. Alternatively, the intramuscular route is effective in the prehospital setting (Silbergleit et al., 2012). In stage two, AEDs were used, but it must be emphasized that there are no clinical trials to inform the best drug treatment at this stage (Cock and ESETT Group, 2011). Phenytoin, levetiracetam, and valproic acid are most often used (Shorvon et al,. 2008). The newer AED lacosamide has also gained acceptance in the community, but data on its effectiveness in convulsive status are limited (Höfler et al., 2011; Trinka, 2011). From stage three onward, intensive care unit treatment and general anesthesia are the mainstays of drug treatment. Again, there are no randomized controlled trials informing the effectiveness of individual drugs in this stage. Midazolam seems to be better tolerated than pentobarbital/thiopental and propofol (Shorvon & Ferlisi, 2012). A fourth stage of status was recently introduced (Shorvon & Trinka, 2011), and a treatment protocol was proposed by Shorvon and Ferlisi (2011). Needless to say, all general measures for intensive care treatment have to be applied at the beginning of status (Shorvon et al., 2008). There is general agreement that AS, dyscognitive status and other forms of focal nonconvulsive SE do not require the same aggressive treatment as convulsive SE. Most patients in AS respond promptly to a benzodiazepine or valproic acid. In focal NCSE the potential risks of treatment, especially intubation and sedation, must be weighed against the benefits of seizure control in preventing neuronal damage and long-term consequences.
Disclosure of Conflict of Interest
AZ has no conflict of interest to declare. JH has received speaker’s honoraria from UCB and travel grants from UCB, Eisai, and Gerot. ET has acted as a paid consultant to Eisai, Medtronics, Bial, and UCB. He has received research funding from UCB, Biogen-Idec, and Sanofi-Aventis, and speakers’ honoraria from Bial, Cyberonics, Desitin Pharma, Eisai, Gerot, Böhringer, Sanofi, Medis, and UCB. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.