Abstract
Iron deficiency anaemia is a global health concern affecting children, women and the elderly, whilst also being a common comorbidity in multiple medical conditions. The aetiology is variable and attributed to several risk factors decreasing iron intake and absorption or increasing demand and loss, with multiple aetiologies often coexisting in an individual patient. Although presenting symptoms may be nonspecific, there is emerging evidence on the detrimental effects of iron deficiency anaemia on clinical outcomes across several medical conditions. Increased awareness about the consequences and prevalence of iron deficiency anaemia can aid early detection and management. Diagnosis can be easily made by measurement of haemoglobin and serum ferritin levels, whilst in chronic inflammatory conditions, diagnosis may be more challenging and necessitates consideration of higher serum ferritin thresholds and evaluation of transferrin saturation. Oral and intravenous formulations of iron supplementation are available, and several patient and disease-related factors need to be considered before management decisions are made. This review provides recent updates and guidance on the diagnosis and management of iron deficiency anaemia in multiple clinical settings.
Graphical Abstract
Introduction
Iron deficiency anaemia (IDA) remains amongst the five leading causes of years lived with disability in humans, and the top cause in women 1. Although it has been mainly regarded as a public health concern affecting growing children, premenopausal and pregnant women, it is also being increasingly recognized as a clinical condition that can affect patients presenting to various medical and surgical specialties, especially those with chronic conditions and the elderly. Emerging evidence on the role of IDA in worsening clinical outcomes continues to accumulate, which prompted careful consideration of IDA diagnosis and management in international practice guidelines. The multiple aetiologies of IDA and nonspecificity of symptoms, however, can challenge the diagnosis. Moreover, the availability of different formulations of iron supplementation can complicate treatment decisions. These aspects are discussed in this Review using the best available evidence and experience of the authors.
Aetiology
There are multiple physiologic, environmental, pathologic and genetic causes of iron deficiency (ID) that lead to IDA (Fig. 1). More importantly, aetiologies may vary considerably or tend to coexist in different patient populations (children, women and elderly), geographies (developing and developed countries) and specific clinical conditions. There is also a considerable complexity and a large repository of terminology for various subtypes of ID which are commonly used interchangeably or in contradiction in the literature.
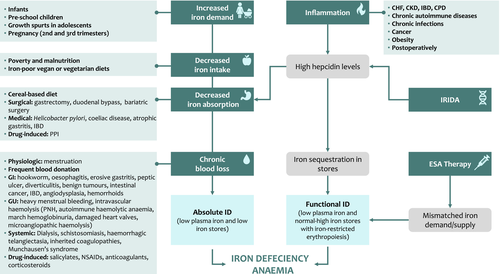
Absolute iron deficiency anaemia
‘Absolute ID’ refers to the reduction of total body iron stores (mostly in macrophages and hepatocytes) which may or may not progress in severity leading to IDA 2. Absolute ID may occur in instances of increased demand, decreased intake, decreased or malabsorption, or chronic blood loss. Increased demand is usually physiologic and commonly noted in infants, preschool children, growth spurts in adolescents and pregnancy (mostly second and third trimesters) 2-4. Decreased iron intake can be a direct consequence of poverty and malnutrition as the case with many children and pregnant women in developing countries or attributed to iron-poor vegan or vegetarian diets 2-4.
Decreased absorption is recognized in certain dietary practices, with several inhibitors of iron absorption recognized such as calcium, phytates (present in cereals) and tannins (present in tea and coffee) 5. It is also attributed to surgical procedures including gastrectomy, duodenal bypass and bariatric surgery (especially Roux-en-Y gastric bypass) which increase stomach pH and decrease conversion to ferrous iron. Certain medical conditions are also known to be associated with decreased iron absorption such as infection with Helicobacter pylori (competition for iron, increased PH and reduction of vitamin C), coeliac disease (gluten-induced enteropathy), atrophic gastritis (increased PH) and inflammatory bowel disease 2-4, 6, 7. It should be noted that such conditions of decreased iron absorption, in most instances, render patients refractory to oral iron therapy 8. The use of proton-pump inhibitors may also contribute to decreased iron absorption 9.
Chronic blood loss can be physiologic as in menstruating women. Frequent blood donors are also known to develop ID 2, 10. In developing countries, hookworm and schistosomiasis infections are very common causes of chronic gastrointestinal or systemic blood loss, respectively 11. In more developed countries, chronic blood loss is mostly observed in women with heavy menstrual bleeding or in men and elderly patients with chronic blood loss from the gastrointestinal tract 2-4. Chronic blood loss is also implicated in patients on dialysis. Other rare causes could include intravascular haemolysis leading to urinary bleeding or other systemic sources of chronic bleeding 2-4. The use of salicylates, nonsteroidal anti-inflammatory drugs, anticoagulants and corticosteroids may also result in drug-induced blood loss due to direct irritation of the gastric mucosa or the tendency of these agents to increase bleeding risk from other causes 2, 4.
Functional iron deficiency anaemia
‘Functional’ or ‘relative’ ID (and subsequent IDA) is a term used in the literature to describe two main scenarios: (i) situations when iron is hardly mobilized from stores to the circulation and erythropoietic tissue in view of chronic inflammation and elevated hepcidin levels, such as in patients with chronic kidney disease, chronic heart failure, inflammatory bowel disease, chronic pulmonary diseases, cancer, obesity, other autoimmune diseases and chronic infections 4. Absolute ID may manifest in later stages in view of decreased iron absorption signalled by high hepcidin levels 3. (ii) Situations of increased erythropoiesis mediated either by endogenous erythropoietin responses to anaemia or by therapy with erythropoiesis-stimulating agents (ESA) creating a mismatch between iron demand and supply 2, 4, 12. It is also common to call erythropoiesis in these instances as iron-restricted or iron-poor erythropoiesis 2, 12. From a diagnostic standpoint, it is essential to realize that indices of iron stores could be normal or high in these scenarios 13.
Iron-refractory iron deficiency anaemia
Genetic causes of IDA exist. Iron-refractory IDA (IRIDA) is a rare recessive condition caused by a mutation in TMPRSS6 leading to elevated hepcidin levels and decreased intestinal iron absorption, a situation similar to the effects of chronic inflammation 14, 15. IRIDA patients are particularly refractory to oral iron supplementation (as well to ESA therapy) 8. Anaemia in IRIDA is usually less severe in adults than in children 16.
Table 1 highlights patient populations and specific clinical conditions for which multiple aetiologies of IDA are commonly observed including children in developing countries, endurance athletes, the elderly, chronic kidney disease, chronic heart failure, obesity, inflammatory bowel disease and following major surgery 2, 4, 6, 17-31. Other nutritional anaemias (folate or vitamin B12 deficiency) may also coexist with IDA, especially in the elderly, children in developing countries or patients with gastrointestinal disorders. Decreased levels of erythropoietin are also reported in elderly patients who may have anaemia in the absence of ID.
| Setting | Aetiologies |
|---|---|
| Children in developing countries |
• Decreased intake (malnutrition) • Chronic gastrointestinal blood loss (parasitic infections) • Decreased absorption (parasitic infections) • Inflammation (from chronic infections)a |
| Endurance athletes |
• Blood loss from haemolysis • Chronic inflammationa |
| Elderly |
• Decreased intake (malnutrition) • Gastrointestinal blood loss (benign or malignant conditions, drug-induced) • Decreased absorption (atrophic gastritis, proton-pump inhibitors) • Chronic inflammatory conditions (including cancer) |
| Chronic kidney disease |
• Chronic blood loss (dialysis, use of anticoagulants) • Decreased intake (malnutrition) • Decreased absorption (proton-pump inhibitors) • Chronic inflammationa • Decreased hepcidin clearanceb • ESA therapyb |
| Chronic heart failure |
• Decreased intake (malnutrition) • Decreased absorption (oedema) • Gastrointestinal blood loss (antiplatelet or anticoagulant) • Chronic inflammationa (in early more than late stages) |
| Obesity |
• Decreased absorption (bariatric surgery, prior to positive effects on weight loss) • Chronic inflammationa |
| Inflammatory bowel disease |
• Decreased intake (malnutrition) • Chronic gastrointestinal blood loss • Decreased absorption (surgical resection in Crohn’s disease) • Chronic inflammationa |
| Major surgery |
• Blood loss • Postoperative inflammation |
- The list includes the most commonly encountered aetiologies although additional risk factors can still coexist
- a Contributing to iron-restricted erythropoiesis (functional iron deficiency anaemia) from high hepcidin levels, and decreased iron absorption in the long term (absolute iron deficiency anaemia).
- b Contributing to iron-restricted erythropoiesis (functional iron deficiency anaemia).
Pathophysiology
Iron homoeostasis
Iron homoeostasis is tightly controlled to avoid the toxic effects of excess iron in the form of harmful reactive oxygen species. Thus, the human body evolved with no means for iron excretion (except for those lost due to cell shedding amounting to around 1 mg per day), with daily absorption limited to 1–2 mg to compensate for daily iron losses. However, the body requires around 25 mg of iron daily, mostly used for the production of haemoglobin in erythrocytes. Iron is also a crucial element for several cellular and tissue functions including respiration, mitochondrial function, energy production, especially in skeletal and cardiac muscles, as well as cell proliferation and DNA repair 3, 32. To achieve this aim, the body recycles most of the required iron from the breakdown of senescent erythrocytes by macrophages in the spleen to make it available to plasma transferrin. This tight control of iron absorption and recycling is mediated through the hepatic hormone hepcidin, but can be easily disturbed leading to various forms of ID and subsequent anaemia 2, 4. Additional recycling mechanisms also exist in skeletal muscle fibres and in hepatic macrophages (in cases of intravascular haemolysis) 33, 34.
Adaptative mechanisms in absolute iron deficiency
Adaptive mechanisms in ID aim to optimize the usage of iron by erythropoiesis and increase its absorption. Hepcidin mainly functions by binding and degradation of its receptor ferroportin on the basolateral membrane of enterocytes and macrophages and thus preventing iron export from these cells into the plasma 35. In absolute ID, hepcidin is suppressed, leading to both increased iron absorption from the gut and release of recycled iron from splenic macrophages into the circulation 35. Hepcidin suppression is triggered by decreases in levels of iron-bound transferrin (ligand of transferrin receptor, noting that non-iron-bound apotransferrin is increased) and hepatic iron stores (unknown mechanism) which lead to increased activity of the inhibitor transmembrane protease serine 6 (TMPRSS6 or matriptase-2) and reduction in the levels of the activator bone morphogenetic protein 6 (BMP6) 2, 36. Epigenetic factors such as histone deacetylase HDAC3 erase activation markers from the hepcidin locus which also contributes to hepcidin suppression.37 Additionally, tissue hypoxia in IDA increases levels of hypoxia-inducible factor 2α (HIF-2α) which stimulates erythropoietin production by the kidney leading to the expansion of erythropoiesis and release of hypochromic, microcytic erythrocytes. This increase in erythropoiesis during anaemia further suppresses hepcidin through the erythroid factor erythroferrone (ERFE), released by erythroblasts 38. HIF-2α also increases the expression of the duodenal divalent metal transporter 1 (DMT1) and duodenal cytochrome B (DCYTB) on the apical surface of enterocytes, thus increasing iron absorption from the gut lumen 39. A role for erythrocyte ferroportin in maintaining plasma iron levels has also been recently reported 40. Once stores are depleted (macrophages followed by hepatocytes), plasma iron levels decrease since iron absorption cannot meet the demand. The recycling of iron from hypochromic erythrocytes in macrophages also decreases in parallel with the severity of ID. Uptake of iron through transferrin receptors is subsequently decreased across all body tissues.
Mechanisms in chronic inflammatory conditions
In conditions associated with chronic inflammation, the mechanism of anaemia is more complex. Chronic inflammation, per se, can lead to a moderate anaemia through several iron-unrelated mechanisms mediated by proinflammatory cytokines – this is called anaemia of chronic disease or anaemia of inflammation which usually resolves when the underlying cause is treated 3, 41, 42.
Chronic inflammation has also been linked to iron dysregulation in recent years 41. The cytokines interleukin (IL)-6, IL1β and IL-22 were shown to increase hepcidin expression leading to ferroportin degradation and sequestration of iron away from the circulation in intestinal enterocytes (eventually being lost through shedding) and macrophages. Stimulation of Toll-like receptors 2 and 6 in chronic inflammation also reduces ferroportin expression in macrophages by hepcidin-independent mechanisms 43. This makes iron less available for use by body tissues in view of lower amounts of plasma iron bound to transferrin and leads to a state of iron-restricted erythropoiesis and anaemia in the presence of normal or increased iron stores 6, 12, 17, 29. As mentioned earlier, in the long-term, this also leads to absolute ID in view of decreased iron absorption 3. It should be noted that in a recent study, mild acute inflammation did not increase serum hepcidin in women with IDA, suggesting low iron status and erythropoietic drive offset the inflammatory stimulus on hepcidin expression 44.
Epidemiology
Anaemia affects one-third of the world population with IDA being the top cause 11, 45. IDA is highly prevalent in preschool children (<5 years), women in the reproductive age and pregnant women with prevalence rates, reaching up to 41.7%, 32.8% and 40.1%, respectively (2016 Global Health Observatory data) 45. Reliance on a vegan diet, malabsorption syndromes and heavy menstrual bleeding are also high-risk categories in high-income countries, with around two-third of women with heavy menstrual bleeding having ID/IDA 2, 11, 46. IDA is more difficult to treat in the elderly population and only represents around 30% of anaemia cases, as other types of anaemia may exist 47. Frequent blood donation is also a poorly recognized cause of IDA. In one study of 2425 individuals, 16.4% and 48.7% of frequent male donors showed the absence of iron stores and iron-restricted erythropoiesis, respectively, with the corresponding proportions of 27.1 and 66.1% for females 48. Although formal estimates on the prevalence of genetic forms of IDA are lacking, IRIDA is thought to represent less than 1% of the cases of IDA seen in medical practice 2.
The reported prevalence of ID/IDA in chronic inflammatory conditions varies greatly amongst different studies, depending on the thresholds of iron parameters used to define ID. ID affects anywhere between 37% and 61% of patients with chronic heart failure 49-53 and between 24% and 85% of patients with chronic kidney disease 54, 55 – higher rates being associated with advanced disease. It is also documented in 13–90% of patients with inflammatory bowel disease depending on disease activity and severity, and whether measured in the outpatient or inpatient setting 54. ID and IDA have also been reported in 42.6% and 33% of cancer patients, respectively, and associated with advanced disease, close proximity to cancer therapy and poor performance status in patients with solid tumours 56. In the surgical setting, preoperative anaemia is present in around one-third of patients undergoing major surgery with over two-third of cases having IDA. ID is also observed in over half of surgical patients without anaemia 57, 58. The prevalence of postoperative anaemia can reach up to 90% 59.
Taken together, these data alert to alarming figures of IDA prevalence across high-risk populations, further highlighting the importance of early detection and management in view of the negative clinical implications.
Clinical Implications
IDA usually develops slowly from the progression of ID. The full range of symptoms and signs associated with IDA at presentation and follow-up are reviewed elsewhere 5, 60, whilst certain clinical consequences are described in this section and Fig. 2. It should be noted, however, that due to nonspecificity of symptoms and the cooccurrence of IDA with multiple morbidities, confounding effects of underlying diseases on the observed association between IDA and outcomes cannot be fully dismissed.
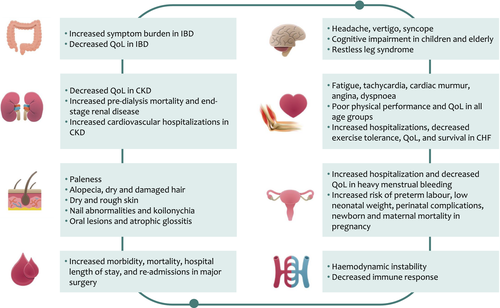
IDA is associated with decreased cognitive performance and delayed motor and cognitive development in children, decreased physical performance and quality of life in adults, especially women in the reproductive age group, and cognitive decline in the elderly 61-63. Although these symptoms remain nonspecific, they can be attributed to low delivery of oxygen to body tissues in IDA. They may also occur as a direct effect of ID 64-67, probably due to reduced iron levels in muscle or brain tissue, and impact on energy production, myoglobin synthesis and brain development. Additional effects of ID attributed to the impact of low iron levels on DNA replication and cell cycle (oral lesions, hair loss, nail abnormalities), immune response (increased susceptibility to infections), myelogenesis and neurotransmission (restless leg syndrome) and inhibition of cytochrome P450 production (altered drug metabolism) are reviewed elsewhere 68.
Women and pregnancy
In women with heavy menstrual bleeding, IDA is associated with reduced quality of life and general well-being, and severe anaemia may lead to hospitalization 69, 70. More importantly, it results in many women entering pregnancy with anaemia. In pregnant women, IDA is associated with an increased risk of preterm labour, low neonatal weight and perinatal complications 69. Severe IDA is also associated with increased newborn and maternal mortality, due to lower tolerance to excessive blood loss during delivery and increased risk of infections 71, 72. Infants born to anaemic mothers are more likely to have IDA themselves 73. ID also carries a negative impact on the mother–child relationship and the child’s cognitive development, an effect measurable for up to 10 years despite iron repletion 74.
Chronic inflammatory conditions
In patients with chronic inflammatory conditions, the impact of IDA can be severe leading to disease exacerbation and deterioration. This is particularly relevant in elderly patients with multiple morbidities, when even mild anaemia can be associated with increased mortality 75.
IDA is a negative prognostic factor in chronic heart failure associated with disease progression, decreased quality of life and an increase in cardiovascular mortality 76, 77. ID without anaemia has also been associated with increased fatigue, reduced exercise intolerance, diminished quality of life, increased hospitalization rates and reduced survival as compared with patients without ID or those who receive iron replenishment 20, 31, 49, 51, 52, 78-81. This is supported by animal studies which show that ID in heart muscles, irrespective of systemic iron levels, is associated with decreased heart contraction, ventricle dilation and heart failure, which can be attributed to the decrease in iron-sulphur cluster synthesis and mitochondrial electron transport in response to stress 82.
In chronic kidney disease, anaemia is commonly associated with reduced energy and diminished quality of life 83, 84. In predialysis patients, there is a cumulative increase in the risk of predialysis mortality or development of end-stage renal disease with decreasing haemoglobin levels (around 2- to 3-fold for haemoglobin values <120 vs. >130 g L−1) 85. An association between the increased risk of mortality and anaemia was also observed in long-term studies irrespective of disease severity 86. Anaemia is also recognized in the context of the cardio-renal anaemia syndrome, and is associated with a two-fold higher rate of cardiovascular hospitalization 87.
Iron deficiency anaemia is the most common extra-intestinal manifestation in inflammatory bowel disease and may affect the quality of life in the same magnitude of other underlying disease-related symptoms such as abdominal pain and diarrhoea 88. In fact, one study showed changes in haemoglobin level are more likely to affect the quality of life than the underlying disease activity 89.
Surgical setting
Preoperative anaemia, even to mild degrees, is associated with an increased likelihood of morbidity and mortality in the 30-day postoperative period after major surgery 58, 59, 90. It is also associated with a longer duration of hospitalization and a higher rate of readmissions 59. These effects have been attributed to the direct effect of anaemia and hypoxia, as well as to the increased risk of perioperative transfusions which themselves are independently associated with an increased risk of postoperative morbidity and mortality 91. Postoperative anaemia has also been associated with infection, poor physical function and recovery, subsequent increases in the length of stay in hospital and mortality 59, 92. Although studies in the surgical setting did not commonly qualify the type of anaemia, the high prevalence of IDA suggests that it may be the main culprit 59.
DIAGNOSIS
General considerations
Three key questions need to be asked when considering the diagnosis of IDA: who should be tested, what tests should be used and what sequence and what laboratory thresholds determine if a patient has IDA?
Since IDA remains the most common cause of anaemia, it should be prioritized in the diagnostic workup of patients presenting with anaemia unless other mechanisms are suspected to be more prevalent in a specific patient. Physicians may need to actively ask high-risk patients about symptoms or signs suggestive of IDA; however, since those may be nonspecific, silent or overlooked, testing patients with certain profiles or underlying diseases known to have high prevalence of IDA, have an established association between IDA and adverse outcomes, and have robust evidence on the clinical benefit of iron treatment from clinical trials may be warranted. This is mostly relevant in patients where multiple causes of IDA may coexist such as in older patients with chronic inflammatory conditions 20, 47. It is also applicable in the perioperative setting for patients undergoing major surgery 59, 92. In young children, studies evaluating screening programmes have found problems with implementation, acceptability and follow-up of testing, and most international authorities do not support this practice. A recent US Preventive Services Task Force recommendation statement concluded that the current evidence base was insufficient to determine the overall net benefit of screening for IDA in asymptomatic children aged 6–24 months. This may not be applicable to infants with additional known risk factors (prematurity) or with symptoms of anaemia 93, 94. The US Preventive Services Task Force also does not recommend screening in asymptomatic pregnant women; however, common practice and expert opinion continue to suggest routine screening for IDA in pregnant women starting the first trimester 69, 71, 93-96. Diagnostic workup should be done for both anaemia and ID simultaneously. When IDA is diagnosed, the continued search for additional causes of anaemia may be necessary in patients with multiple mechanisms of anaemia such as the elderly, chronic inflammatory conditions or patients with malnutrition 20, 47. Evaluation of the cause(s) of IDA is warranted since if it can be actively treated, it will aid in the resolution of IDA beyond iron therapy 2, 5. Of course, if the diagnostic workup reveals anaemia but no ID, further assessment of alternate causes should be made to establish the optimal course of therapy. However, in high-risk groups such as infants, preschool children, growing adolescents, young females and pregnant women, extensive diagnostic workup is not needed considering the cause of IDA is often physiologic in view of increased iron demand or loss and may be limited to instances of lack of response to therapy. In patients determined to be eligible for diagnostic evaluation but show no laboratory evidence of IDA, reassessment can be done at subsequent visits depending on the underlying condition and its activity. Figure 3 provides an algorithm for the diagnostic workup of IDA. Detailed diagnostic workups in specific clinical settings and populations such as women’s health and pregnancy 69, 71, 95, elderly 21, 22, children 54, chronic inflammatory conditions 20 and perioperatively 59, 92 have been reviewed elsewhere.
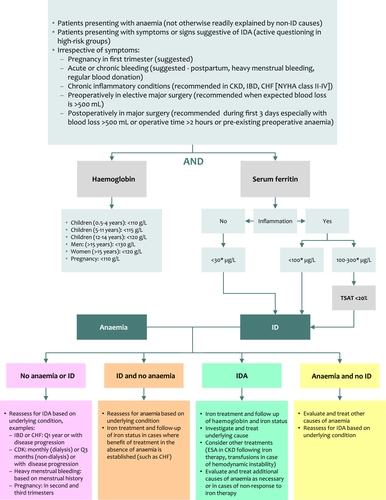
Laboratory tests and thresholds
The diagnosis of IDA can be readily made by assessing haemoglobin and serum ferritin levels. According to the World Health Organization (WHO), anaemia is defined as a haemoglobin level <130 g L−1 in men, <120 g L−1 in nonpregnant women and <110 g L−1 in pregnancy 97. Specific thresholds at various stages of childhood are also commonly used (Fig. 3) 97. Further categorization within the various stages of pregnancy is provided by the Centres for Disease Control and Prevention (CDC): first and third trimesters <110 g L−1, second trimester <105 g L−1 and postpartum <120 g L−1 98. That said, it is worth noting that a recent WHO technical meeting concluded that there are minimal data to support haemoglobin thresholds to define anaemia. Future directions in assigning more evidence-based thresholds could have considerable implications on disease definition, epidemiology and management 99. Serum ferritin level is the most specific and effective test to reflect total body iron stores and is universally available and standardized 4. Although a value <12–15 μg L−1 is confirmatory for ID, a value of <30 μg L −1has higher sensitivity (92%) and similar specificity (98%) and is more widely used 5, 100, 5, 100. Although widely implemented and cited, these cutoffs are based on qualitative expert opinion and not a systematic appraisal of the published work. Several projects are being coordinated by the WHO and CDC to address this evidence gap; until then, clinicians can rely on laboratory standards at their centre and local guidelines 101. In the presence of inflammation, interpretation of serum ferritin level is more challenging. First, ferritin, in the form of apoferritin, is an acute-phase reactant that increases in inflammation. Second and as explained earlier, in chronic inflammatory conditions the increase in hepcidin levels leads to iron sequestration in macrophages. This is reflected with normal or even high serum ferritin levels despite decreased availability of iron in the circulation. Although this has created considerable variability in recommended serum ferritin thresholds for the diagnosis of ID during inflammation between guidelines (<50, <100 or <200 μg L−1) 20, 54, 59, 69, 92, a recent international expert consensus recommended using serum ferritin levels <100 μg L−1 to diagnose ID in chronic inflammatory conditions 20, and the threshold is also commonly recommended in the elderly and postoperatively 4, 92. Additionally, during inflammation serum iron is still reduced and total iron-binding capacity is increased despite normal or high iron stores (functional ID), which leads to a substantial reduction in transferrin saturation (ratio of serum iron to total iron-binding capacity). A transferrin saturation <16% is commonly used to diagnose ID in general and a threshold of <20% is proposed in the presence of inflammation 5, 20. Thus, a practical approach during inflammation would be to consider serum ferritin <100 μg L−1 as diagnostic whilst for patients with values of 100–300 μg L−1 to check transferrin saturation and diagnose ID in those with values <20%. Although this sequential approach is probably more costly, it may be more practical to test for serum ferritin and transferrin saturation at the same time to avoid repeated blood withdrawals and laboratory visits. Although identifying a state of inflammation is straight forward in chronic inflammatory conditions or postoperatively, for example, confirmation through evaluation of markers of inflammation may be needed in equivocal scenarios or when the intention is to wait for acute inflammation to resolve before relying on iron values. A C-reactive protein value of >5 mg L−1 is commonly used in the setting of acute inflammation, although not widely standardized 20, 54, 59, 69, 92. Alpha glycoprotein (AGP) may also be used as an indicator of chronic inflammation 102.
Other laboratory tests
In IDA, red cells are microcytic and hypochromic, as shown by low mean corpuscular volume (MCV) and mean corpuscular haemoglobin (MCH) and increased red blood cell distribution width (RDW). However, changes in red cell indices occur late in the course of IDA in view of the red cell lifespan, and the usefulness of these tests may be limited. Reticulocyte haemoglobin content (RHC) indicates the amount of iron available for erythropoiesis in the previous 3–4 days and is an early index of ID; the rapidity of changes makes it a potentially useful marker to identify responders to iron supplementation. The percentage of hypochromic red cells (% HRC) also reflects recent iron reduction, yet the value of these tests in long-standing IDA is limited and they are not extensively used in clinical practice for the diagnosis of IDA 3.
Serum soluble transferrin receptor (sTFRC) levels are increased in ID (and normal/low in inflammation). A meta-analysis showed a high sensitivity (86%) and specificity (75%) for sTFRC in the diagnosis of ID, but this remained lower than that of serum ferritin 103. The ratio between sTFRC and log ferritin (sTFRC–ferritin index) may help in recognizing IDA in the setting of chronic inflammation, although lack of standardization between available assays limits wide use in clinical practice 41.
Hepcidin level is decreased/undetectable in IDA, but it is affected by several factors including circadian rhythm, hepatic and renal function 3. Hepcidin assessment may be useful to confirm IRIDA, because levels are constitutionally high or normal 18. Few studies also suggested that high hepcidin levels may be useful in predicting response to oral iron supplementation 104, 105. Although a good correlation between different methods of hepcidin level assessment is observed 106, it is still not routinely or widely used in clinical practice.
In patients with IRIDA, typical findings include a striking microcytosis and extremely low transferrin saturation in the presence of normal or borderline low serum ferritin levels 4.
MANAGEMENT
Prevention
Efforts to increase access to and consumption of iron-rich foods should always be in place. Iron absorption enhancers (ascorbic acid) or inhibitors (calcium, phytates [cereals], tannins [tea and coffee]) should also be considered when supplying iron-rich meals. Enrichment of food (rice, maize flour, cornmeal) with iron is also practiced in some countries, such as in Asia, Africa and Latin America, and recommended by the WHO 5, 107, 108. The WHO recommends iron supplementation to prevent ID/IDA in instances where the prevalence of anaemia is 40% or higher: children 6–23 months (10–12.5 mg elemental iron daily – drops/syrups, three consecutive months in a year), 24–59 months (30 mg elemental iron daily – drops/syrups/tablets, three consecutive months in a year), 5–12 years (30–60 mg elemental iron daily – tablets/capsules, three consecutive months in a year). In malaria-endemic areas, the provision of iron supplementation in infants and children should be done in conjunction with public health measures to prevent, diagnose and treat malaria 109. Although iron repletion can reverse the protective effect of ID on malaria infection, it is less of a concern in areas where preventive services are provided 110, 111. The WHO also recommends iron supplementation in the same setting for menstruating adult women and adolescent girls (nonpregnant females in the reproductive age group) with 30–60 mg elemental iron daily tablets for three consecutive months in a year 112.
Iron supplementation for iron deficiency anaemia
General considerations
Management of the underlying cause of IDA should always be considered. For example, treating H. pylori infection or introduction of gluten in coeliac disease can restore iron absorption 8, 113. Investigation and management of the source of gastrointestinal blood loss are also warranted in men and postmenopausal women. These measures not only prevent further iron loss or improve iron absorption, but also aid in prompt diagnosis of potentially serious underlying diseases.
An important question in IDA management is whether oral or intravenous iron therapy should be used. Comparison of both modes of administration is summarized in Table 2 20. Generally speaking, factors that should be considered in decision making include patients’ age and sex, the underlying condition and cause of IDA, the severity of anaemia or ID and their symptoms, and the time frame available or acceptable for correction. Intravenous therapy can be generally considered when response, tolerability or adherence to oral iron therapy are not ideal or when anaemia or ID is severe. Unless contraindicated, oral iron therapy can be started and patient evaluated with haemoglobin and iron indices; the frequency of monitoring and duration of therapy should rely on the underlying condition and specific treatment goals. In cases of intolerance or when signs of nonresponse are noted despite efforts to improve tolerance (low or alternate day dosing, use of newer oral formulations 4, 114, 115), adherence (modification of dose and frequency) or absorption (eradication of H. pylori infection or introduction of gluten-free diet in coeliac disease), intravenous iron can be considered with appropriate dosing. Continued monitoring of haemoglobin and iron status every 1–3 months should be ensured until normalization of laboratory values; whilst further investigation for alternate causes of anaemia is warranted in case of nonresponse 5. More specific indications for intravenous iron therapy are summarized in Table 3 and relevant data is further discussed in this section. Six intravenous iron formulations are available: ferric gluconate, iron sucrose, low-molecular-weight iron dextran, ferumoxytol ferric carboxymaltose and iron isomaltoside. Details of various formulations, their dosing and pivotal data on efficacy, safety and adverse event management are reviewed elsewhere 2, 4, 5, 116, 117, but use should follow local guidelines and product prescribing information.
| Parameter | Oral | Intravenous |
|---|---|---|
| Absorption and bioavailability |
• Ingested iron absorption is low (10–20%) • Reduced in inflammation conditions |
• Not affected by inflammation |
| Administration ease |
• Easy |
• Requires expertise and facility for cardiopulmonary resuscitation • Administration site reactions •Potentially fatal hypersensitivity reactions (especially iron dextran) • Recurrent dosing |
| Dosing |
• Daily up to three times per day |
• Single high-dose or multiple |
| Response |
• Can be limited |
• Higher efficiency and shorter duration to improve haemoglobin levels and iron indices |
| Gastrointestinal side effects |
• Higher |
• Lower |
| Other side effects of interest |
• Skin discoloration |
• Headache and joint pain • Hypophosphataemia and osteomalacia with some formulations |
| Adherence |
• Lower |
• Higher |
| Cost |
• Lower |
• Higher |
| Overdose |
• Accidental overdose possible with ferrous salts |
• Variable complex stability, can induce oxidative stress at high doses |
| Indication |
|---|
| Intolerance, nonresponse, poor adherence to oral iron |
| Rapid or significant correction of anaemia and/or iron deficiency needed |
| Medical and surgical conditions with decreased absorption (cause cannot be treated) |
| Iron-refractory iron deficiency anaemia (IRIDA) |
| Chronic heart disease (systolic, NYHA class II–IV) |
| Chronic kidney disease (dialysis, or with ESA treatment) |
| Inflammatory bowel disease (active disease or haemoglobin <100 g L−1) |
| Preoperatively (surgery scheduled in <6 weeks) |
| Postoperatively |
| Pregnancy (second trimester if haemoglobin <105 g L−1 and third trimester) |
- ESA, erythropoiesis-stimulating agents; NYHA, New York Heart Association.
Pregnancy
In pregnant women with IDA, recent guidelines recommend use of intravenous iron in the second trimester if haemoglobin level is <105 g L−1 and anytime in the third trimester 71. Earlier studies indicated comparative effects of intravenous and oral iron in pregnant women, with respect to haematologic, maternal and foetal outcomes 118. More recent trials, however, showed the superiority of intravenous over oral iron during pregnancy 119, 120. A single dose of intravenous iron was also more effective than oral iron in improving fatigue after postpartum haemorrhage, and was as effective as blood transfusions in management of severe anaemia; although the latter would be needed in cases of haemodynamic instability 121, 122. Risk-benefit and cost-effectiveness considerations should always be taken into consideration before the decision to use intravenous iron therapy is made, especially in resource-poor countries.
Chronic inflammatory conditions
In patients with chronic inflammatory conditions, response to oral iron therapy may be limited by the hepcidin-mediated decreased absorption. In iron-deficient patients with heart failure and reduced ejection fraction, high-dose oral iron did not improve exercise capacity over 4 months 123. In placebo-controlled trials, intravenous iron therapy significantly improved symptoms, functional capacity and quality of life in patients with chronic heart failure [New York Heart Association (NYHA) class II or III] and ID, irrespective of the presence of anaemia 77, 124. A reduction in hospitalization rate by up to 61% was also observed in other trials 125. A recent meta-analysis of randomized trials concluded that intravenous iron therapy in patients with systolic heart failure and ID improves outcomes, exercise capacity and quality of life, and alleviates heart failure symptoms 126. This has led to wide recommendation of compulsory screening and intravenous iron treatment in chronic heart failure patients even in the absence of anaemia 20. It should be noted, however, that the previously mentioned thresholds to define ID (using ferritin and TSAT) which are also commonly used in these trials have rarely been prospectively validated 127.
In inflammatory bowel disease, gastrointestinal side effects, increased local inflammation from iron and alterations in gut microbiota can mitigate the use of oral iron therapy 128. Laboratory or imaging studies may be needed to determine disease activity and decide on the choice of oral or intravenous iron therapy. In patients with active or advanced disease or those with haemoglobin values ≤100 g L−1, intravenous iron is recommended over oral iron for the management of IDA 20, 129. Unfortunately, the need for iron replenishment in these patients is poorly recognized with one study showing that over 50% of patients with inflammatory bowel disease and IDA did not receive treatment 130.
In patients with chronic kidney disease, oral or intravenous iron may be used, although data from clinical trials show better haemoglobin response in patients with IDA who took intravenous compared with oral iron 131. Normalization of haemoglobin levels is associated with significant physical function improvement, but the effect on the risk of cardiovascular events is not clear.111 Intravenous iron is recommended front line in patients on dialysis 20. A recent trial showed that in patients undergoing haemodialysis, proactive administration of high-dose intravenous iron is superior to reactive administration (triggered by a diagnosis of ID) of a low-dose regimen and resulted in lower doses of ESA being used 132. ESA therapy is not always associated with clinical benefit in patients with chronic kidney disease, and anaemia and may be associated with increased risk of stroke 133. When administered, ESA therapy should always follow iron treatment since it may worsen ID, and in this setting intravenous iron is more effective than oral iron 20. Iron therapy may also delay or abolish the need for ESA in the first place 19. In cancer patients, iron therapy is recommended for IDA or following a lack of response to ESA 19. An increased risk of infection with the use of intravenous iron, especially in haemodialysis patients, has been a subject of debate. A recent meta-analysis concluded that high-dose parenteral iron does not seem to be associated with higher risk of infection or all-cause mortality on analysis of randomized trials. Observational studies show increased risk for all-cause mortality, infections and hospitalizations that were not statistically significant and were associated with significant heterogeneity 134.
Cancer
Iron administration to anaemic cancer patients can increase the level of haemoglobin probably independently from the specific mechanism of anaemia, which may help reduce the need for blood transfusions and ESA therapy 135. There is a lack of consensus on the specific indication for iron supplementation, although most guidelines recommend use when ID is documented, and a preference towards intravenous iron therapy especially in cases of functional IDA or with planned use of ESA 136, 137.
Surgical setting
In surgical patients, the concept of patient blood management is widely recognized 138, and relies on the idea of early detection of anaemia and management prior to major surgical procedures to avoid the need for blood transfusions perioperatively, the latter being strictly reserved to patients with severe anaemia and haemodynamic instability. The consensus is that IDA needs to be identified and treated as soon as the decision to perform an elective surgery is taken, otherwise, surgery may be delayed if possible until IDA is effectively managed. International guidelines recommend that preoperative IDA can be treated with oral iron therapy, whilst intravenous iron can be used in case of nonresponse or intolerance, or front line if surgery is scheduled within less than six weeks 59. Recent data from clinical trials, however, show superiority of intravenous over oral iron in improving haemoglobin level and iron indices 139, and effectiveness of intravenous iron in decreasing the duration of hospital stay, postoperative anaemia and need for blood transfusions 140-142. Safety was also established in such trials. This may suggest that the use of intravenous iron in the preoperative setting could be prioritized, not only when the time available for correction of IDA is short, but also in patients with severe IDA or patients that are known to be less responsive to oral therapy such as older patients with chronic inflammatory conditions. In the postoperative period, early intravenous iron therapy is recommended as a single high dose unless otherwise contraindicated, especially in view of postoperative inflammation 92. In patients with IDA, intravenous iron therapy was associated with improvement of anaemia and ID, fewer blood transfusions and lower risk of severe infections in the four weeks following surgery 143, 144.
Conclusion
Iron deficiency anaemia is a significant public health concern that can cause debilitating clinical consequences across age groups, genders, geographies and clinical conditions. Early diagnosis and effective management are thus needed to avoid associated sequelae. This can only be achieved with increased awareness of the prevalence and causes of IDA, as well as the benefits of treatment, amongst healthcare professionals. Although effective means for iron supplementation exist, making the right and timely choice between oral and intravenous iron formulations is essential to avoid unnecessary delays in iron repletion and correction of anaemia. The development of new oral iron formulations with fewer gastrointestinal side effects may result in more patients receiving and tolerating therapy. Moreover, many studies of intravenous iron have limited follow-up duration or small sample sizes, and large, randomized trials with standardized end points are needed to better establish the role of intravenous therapy across indications. Ongoing research aiming to better understand iron metabolism and the role of hepcidin as a diagnostic tool and treatment target could bring more novel diagnostic and therapeutic interventions in future years.
Acknowledgements
We apologize to those authors whose work could not be cited in this review due to space limitations.
Conflicts of interest
MDC, KMM and ATT received honoraria from Vifor.
Funding source
None.
Authors contributions
MDC, KMM and ATT participated in literature search, manuscript drafting and review. All authors approved the final manuscript prior to submission.





