Determining reinfection rates by hepatitis C testing interval among key populations: A systematic review and meta-analysis
Stephanie C. Munari and Michael W. Traeger should be considered joint first author.
Handling Editor: Aghemo Alessio
Abstract
Background & Aims
Detecting hepatitis C virus (HCV) reinfection among key populations helps prevent ongoing transmission. This systematic review aims to determine the association between different testing intervals during post-SVR follow-up on the detection of HCV reinfection among highest risk populations.
Methods
We searched electronic databases between January 2014 and February 2023 for studies that tested individuals at risk for HCV reinfection at discrete testing intervals and reported HCV reinfection incidence among key populations. Pooled estimates of reinfection incidence were calculated by population and testing frequency using random-effects meta-analysis.
Results
Forty-one single-armed observational studies (9453 individuals) were included. Thirty-eight studies (8931 individuals) reported HCV reinfection incidence rate and were included in meta-analyses. The overall pooled estimate of HCV reinfection incidence rate was 4.13 per 100 per person-years (py) (95% confidence interval [CI]: 3.45–4.81). The pooled incidence estimate among people who inject drugs (PWID) was 2.84 per 100 py (95% CI: 2.19–3.50), among men who have sex with men (MSM) 7.37 per 100 py (95% CI: 5.09–9.65) and among people in custodial settings 7.23 per 100 py (95% CI: 2.13–16.59). The pooled incidence estimate for studies reporting a testing interval of ≤6 months (4.26 per 100 py; 95% CI: 2.86–5.65) was higher than studies reporting testing intervals >6 months (5.19 per 100 py; 95% CI: 3.92–6.46).
Conclusions
HCV reinfection incidence was highest in studies of MSM and did not appear to change with retesting interval. Shorter testing intervals are likely to identify more reinfections, help prevent onward transmission where treatment is available and enable progress towards global HCV elimination, but additional comparative studies are required.
Abbreviations
-
- AASLD
-
- American Association for the Study of Liver Diseases
-
- cAg
-
- core antigen
-
- DAA
-
- direct-acting antiviral
-
- EASL
-
- European Association for the Study of the Liver
-
- GRADE
-
- Grading of Recommendations, Assessment, Development and Evaluations
-
- HCV
-
- hepatitis C virus
-
- MSM
-
- men who have sex with men
-
- OAT
-
- opioid agonist therapy
-
- PrEP
-
- pre-exposure prophylaxis
-
- PWID
-
- people who inject drugs
-
- PYFU
-
- person-years of follow-up
-
- RCTs
-
- Randomised controlled trials
-
- RNA
-
- ribonucleic acid
-
- SVR
-
- sustained virologic response
-
- TasP
-
- treatment-as-prevention
-
- WHO
-
- World Health Organisation
Key points
- Thirty-eight studies (8931 individuals) reported HCV reinfection incidence rate and were included in meta-analyses.
- The overall pooled estimate of HCV reinfection incidence rate was 4.13 per 100 per person-years (py).
- HCV reinfection incidence was highest in studies of MSM (7.37 per 100py) compared with PWID (2.84 per 100py) and people in custodial settings (7.23 per 100 py).
- HCV reinfection incidence was similar among studies that tested at >6-month intervals (5.19 per 100 py) compared with studies reporting testing at ≤6-month intervals (4.26 per 100 py) though findings were not statistically significant.
- HCV reinfection incidence did not appear to change with retesting interval. Longitudinal studies comparing annual HCV retesting with more frequent retesting among key populations are required.
1 INTRODUCTION
Global elimination targets set by the World Health Organisation (WHO) aim for the elimination of viral hepatitis as a public health concern by 2030. To achieve elimination, targets call for the treatment of 80% of those eligible and a 90% reduction in incidence of new hepatitis B and C infections by 2030 compared with 2015 levels.1 Only 12 of 194 countries are reported to be on track to meet the targets,2 perhaps even fewer at the time of writing with a significant reduction in hepatitis C virus (HCV) testing and treatment rates during the COVID-19 pandemic.3 Worldwide, HCV was responsible for over a quarter of the 1.1 million deaths caused by viral hepatitis in 2019, due largely to chronic liver disease and liver cancer.1 Incidence of HCV infection is highest among key populations, with 39% of the 1-year global population attributable fraction of HCV transmission in 2018–19 associated with intravenous drug use.4 High incidence of HCV has also been observed in studies of men who have sex with men (MSM) living with HIV and those using HIV pre-exposure prophylaxis (PrEP).5 Microelimination programmes targeting key populations suggest that it may be possible to reduce HCV incidence by improving linkage post-diagnosis to care and treatment, to reduce the risk of reinfection or diagnose and treat it at the earliest possible time.6-11
Treatment-as-prevention (TasP), where risk of onward HCV transmission is lowered through high treatment coverage and reduced prevalence, is a key pillar of global elimination efforts and associated reductions in chronic liver disease-related morbidity and mortality.12 Modelling has suggested that to achieve the WHO HCV incidence reduction targets, more frequent testing is needed in high-prevalence settings.13 While the advent of highly effective and tolerable treatments, known as direct-acting antiviral (DAA) medication, has led to approximately 9.4 million people with HCV infection being treated between 2015 and 2019 worldwide,1 individuals may remain at risk of reinfection following cure. Reinfection is defined as recurrent viraemia after its clearance either spontaneously or as a result of treatment.14 People who inject drugs (PWID), MSM and people in custodial settings are among those at highest risk of recurrent viremia.15-17 Guidelines recommend ‘focused’ testing in these populations, along with ongoing linkage to prevention and care services, and suggest targeted testing among these populations is likely to be cost-effective.15 Following SVR, the European Association for the Study of the Liver (EASL) recommend at least annual, preferably biannual monitoring for HCV reinfection among PWID and MSM18 and the American Association for the Study of Liver Diseases (AASLD) recommend annual RNA testing among patients with ongoing risk including intravenous drug use or MSM engaging in unprotected sex.17 This systematic review was commissioned by the WHO to inform their ‘Consolidated guidelines on HIV, viral hepatitis and STI prevention, diagnosis, treatment and care for key populations’, where key populations included MSM, PWID, people in prisons and closed settings, sex workers and trans and gender diverse people. We aimed to provide specific additional evidence on the association between different reinfection testing intervals and the detection of HCV in the post-SVR follow-up period among highest risk populations.
2 MATERIALS AND METHODS
This systematic review was commissioned and guided by the WHO Global Hepatitis Programme. The review protocol was prospectively registered with PROSPERO (CRD42021249863).
2.1 Study identification
Three electronic databases (MEDLINE, Embase and Web of Science) were searched for studies published between 1 January 2014 and 1 February 2023 in preparation for the 2022 WHO global testing recommendations for key populations. Search terms included ‘hepatitis C’, ‘HCV’, ‘test’, ‘screen’, ‘antigen’, ‘RNA’, ‘cAg’, ‘reinfection’ and ‘infection’. The full search strategy is outlined in Appendix A. Abstract repositories from relevant international conferences, including The International Liver Congress, The Liver Meeting, The International Conference on Hepatitis Care in Substance Users, The International Symposium on Viral Hepatitis and Liver Disease, The International AIDS Conference, The International AIDS Society Conference on HIV Science and Conference on Retroviruses and Opportunistic Infections, from 2014 to 2020 were also searched. Citation lists of included articles were manually reviewed to identify additional articles that met inclusion criteria.
2.2 Study selection
Search results were uploaded to Covidence and study titles and abstracts were each independently assessed by at least two reviewers (SM, MT, VM).
2.3 Eligibility criteria
Studies were included if they sampled people with evidence of cleared previous HCV infection (spontaneous clearance or cured) and who were tested for reinfection with a HCV ribonucleic acid (RNA) or core antigen (cAg) test. Studies were included if participants were according to the following key populations: MSM, PWID, transgender people and people in custodial settings, as these populations are among those at highest risk of HCV reinfection. For this review, studies of PWID were those which included participants reporting to be currently or recently injecting drugs, as well as those receiving opioid agonist therapy (OAT). Studies were included if testing for HCV reinfection were scheduled to occur at discrete intervals of up to every 12 months. Studies that tested individuals at variable testing intervals or at clinician's discretion were not included. Randomised controlled trials (RCTs), comparative observational studies and one-armed observational studies published in English from any country were eligible for inclusion. Studies were included if they reported on the primary outcome: detection of new HCV infections. Data for a range of secondary outcomes related to test uptake, linkage to treatment following reinfection and adverse events were also extracted from included studies.
- Studies with less than 15 participants in total.
- Studies that included children (defined as persons under 18 years of age).
- Review studies and case study papers.
- Studies whose observation period ended before January 2014 as studies prior to this year were not within the direct-acting antiviral (DAA) era.
- Studies that did not perform HCV testing at discrete time intervals.
2.4 Data extraction
Two independent reviewers extracted data from each study using a standardised spreadsheet and discrepancies were reviewed through discussion and involvement of a third reviewer. Where outcome data were missing or incomplete, study authors were contacted for additional data, with a minimum of two attempts. Where a study resulted in multiple publications, the most up-to-date and comprehensive data were included. The following data were extracted: country, study cohort or setting, study design, sample size, definition and proportion of PWID, MSM, transgender people and people in custodial settings, proportion of cohort with HIV coinfection, treatment regime for post-treatment studies, start and end date of follow-up, testing frequency, number of reinfection cases, person-years of follow-up for reinfection, and incidence of HCV reinfection per 100 person-years and upper and lower confidence intervals were reported.
2.5 Data synthesis and analysis
Studies which reported the HCV reinfection as an incidence rate per 100 person-year were included in the meta-analysis. Random-effects meta-analysis was used to estimate a pooled HCV reinfection incidence rate. Where incidence rates or confidence intervals were not reported, they were calculated when sufficient data were reported. Statistical heterogeneity between studies was quantified by calculating an I2 statistic and χ2 value, with an I2 > 50% considered as moderate/high heterogeneity.
Pooled estimates were disaggregated by cohort risk group (PWID, MSM and people in custodial settings) and testing interval (testing intervals less than or equal to 6 months versus longer than 6 months) to investigate sources of heterogeneity and compare differences in pooled incidence rates between groups. The ≤6 month and >6 month testing interval dichotomy was decided post-hoc based on the observed variation of testing intervals of included studies. Studies with testing intervals that changed over time were allocated to the testing category that most closely resembled most tests performed. All statistical analyses were performed with Stata 15 (StataCorp).
2.6 Risk of bias of individual studies
A modified Newcastle-Ottawa Scale (Appendix B) was used to assess the risk of bias in the included one-armed observational studies. Risk of bias in individual studies was assessed based on selection and outcome characteristics and was classified using a numerical scale from zero to two for each criterion, with a maximum total score of nine. A score of seven or greater was classified as low risk of bias.
3 RESULTS
3.1 Search results
A total of 14 408 citations were identified from the search strategy, of which 8140 were duplicates. Of the 6268 unique citations screened for eligibility, 220 were eligible for full-text review (Figure 1). A further 11 studies were identified for full-text review by searching conference abstracts and reference lists of included studies. Of the 231 full texts screened, 190 were excluded (study exclusion reasons outlined in Figure 1). The most common reasons for exclusion related to study design, including studies which did not test individuals at discrete testing intervals (n = 91) and studies that sampled populations other than the populations in our inclusion criteria (n = 14).
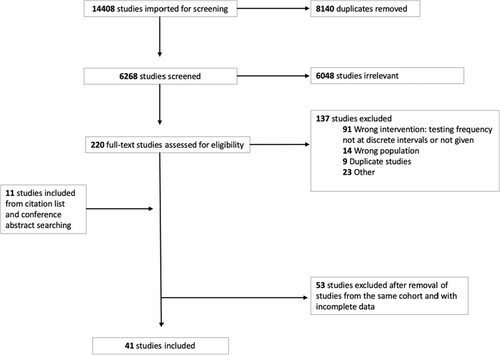
3.2 Included Studies
Forty-one studies were included in the review, all of which were one-armed observational studies; no RCTs or comparative observational studies were identified. Characteristics of included studies are outlined in Table 1. The 41 observational studies included 8931 participants at risk of HCV reinfection. Thirty-five studies were from high-income countries, five from upper-middle income countries and one from both upper middle- and high-income countries as a multi-centre cohort. All 41 studies reported the detection of new HCV reinfections and no studies reported on the secondary outcomes of test uptake, linkage to treatment following reinfection or adverse events.
| Study | Study cohort and setting | World Bank income group (2020)1 | Study design | Study population | Total cohort sample size | Start date of follow-up | Duration of follow-up | Testing frequency | Assigned testing interval category |
|---|---|---|---|---|---|---|---|---|---|
| Aitken et al 201736 |
MIX—Melbourne Injecting Drug Users Cohort Study, Melbourne, Australia |
High income | Prospective | PWID | 757 | November 2008 | — | 12 months | >6 |
| Akiyama et al 202037 |
PREVAIL Montefiore General clinical Research Centres or 1 of 3 OAT clinics, Bronx, New York |
High income | Prospective |
PWUD 75% PWID |
141 | April 2017 | Median 20.5 months | 6 months | ≤6 |
| Baxter et al 201838 |
North Manchester Hospital database, Manchester, UK |
High income | Prospective | PWID 100% | 45 | Mean 50 months (range 11–95 months) | 2 visits in total at least 1 year apart | >6 | |
| Berenguer et al 201919 (MSM) |
Madrid Coinfection Registry (Madrid-CoRe), Madrid, Spain |
High income | Prospective | MSM 7% | 177 | November 2014 | Median 15 weeks post-SVRb | One-off 3 months, and then every 6–12 months | >6 |
| Berenguer et al 201919 (PWID) |
Madrid Coinfection Registry (Madrid-CoRe), Madrid, Spain |
High income | Prospective | PWID 62% | 1459 | November 2014 | Median 15 weeks post-SVR | One-off 3 months, and then every 6–12 months | >6 |
| Bregenzer et al 202239 | Outpatient Centre for Opioid Agonist Therapy, and Department of Infectious Diseases and Hospital Hygiene, Cantonal Hospital, Switzerland | High income | Prospective | PWID | 19 | April 2018 | Median 1.8 years | Monthly | ≤6 |
| Buscillao et al 201840 | Needle Syringe Program, Tbilisi, Georgia | Upper middle income | Prospective |
PWID 100% 57% recent drug use 56.8% use in last 6 months |
169 | July 2015 | Median 12.3 months | At month 6 and month 12 | ≤6 |
| Byrne et al 202041 |
NHS Tayside, Scotland |
High income | Retrospective |
PLWHIV 80% PWID 10% Sexual transmission |
44 | January 2001 | Median 7 years (IQRa 2–12) | 12 monthly or ad hoc if raised ALT | >6 |
| Byrne et al 202242 |
NHS Tayside, Scotland |
High income | High income | PWID | 227 | January 2017 | 256.57py | 12 monthly | >6 |
| Carson et al 202243 |
STOP-C study Australia |
High income | Prospective | Prisoners | 161 | October 2014 | 145py | 3–6 monthly | ≤6 |
| Chen et al 202220 | National Taiwan University Hospital, Taiwan | Upper middle income | Retrospective |
PLWHIV 83.5% MSM 10.6% IDUs |
284 | January 2018 | Median 2.32 years | 3–6 monthly | ≤6 |
| Cheng et al 202244 | HIV care hospital, Taiwan | Upper middle income | Retrospective |
PLWHIV 78.9% PWID 20.3% MSM |
516 | June 2009 | Median 63.6 weeks | 12 monthly | >6 |
| Coffin et al 201945 |
BYE-C, US |
High income | Prospective | PWID | 31 | 2015 | — | Week 2, 4, 8 of Tx. Week 1, 12, 36 post-Tx (= at 3 months, 9 months) | ≤6 |
| Cotte et al 201846 |
Dat'AIDS, France |
High income | Prospective | MSM | 11 467 | January 2016 | — | 3–6 months | ≤6 |
| Cunningham et al 202147 |
SIMPLIFY and D3FEAT, 8 countries |
High income | Prospective |
PWID All recent IDU or current OAT |
190 | March 2016 | Median 1.8 years | SVR 12, SVR24, 60w, 84w, 108w (= at 3, 6, 15, 21, 27 months) | ≤6 |
| Doyle et al 201948 |
TAP, Australia |
High income | Prospective | 100% PWID within last 6 months | 241 | — | — | 3 monthly | ≤6 |
| Farley et al 201849 | Community-based clinic that also services correctional institutions, Canada | High income | Retrospective | Prisoners | 132 | January 2000 | >/=10 years | 6 months | ≤6 |
| Forns et al 202050 | Harm reduction and addiction centres, Catalonia, Spain | High income | Retrospective | PWID | 20 822 | — | — | 12 weeks, 36 weeks and 60 weeks after end of therapy. | ≤6 |
| Foschi et al 202151 | 6 outpatient clinics in Emilia-Romagna, Italy | High income | Prospective | PWID | 338 | May 2015 | Median 53 weeks | 6 monthly | ≤6 |
| Grebely et al 202252 |
CO-STAR, Multi-country |
Upper middle and high income | Prospective | PWID – people receiving OAT | 286 | July 2015 | 604py | 6 monthly | ≤6 |
| Gonzalez-Serna et al 202021 |
Four hospitals, Southern Spain |
High income | Prospective | MSM | 350 | January 2016 | Median 34.9 months (20.7–37.7 IQR) | 12 months | >6 |
| Holeksa et al 201953 |
Vancouver Infectious Diseases Centre, Vancouver, Canada |
High income | Retrospective | PWID | 243 | March 2014 | Median 714 days (range 134–1841 days) | 6 months | ≤6 |
| Hoorenborg et a 202054 | Amsterdam PrEP study, Netherlands | High income | Prospective |
MSM 99% TGW 1% |
350 | August 2015 | 653.6 days | 6–12 months | >6 |
| Huang et al 201955 |
National Taiwan University Hospital, Taiwan |
Upper middle income | Retrospective | MSM 90% | 225 | January 2011 |
Median 4.4 years (IQR 2.8–6.6) for reinfection 3.1 years (IQR 2.1–5.2) for no reinfection |
Median 5.7 months (IQR 2.7–9.6) | >6 |
| Ingiliz et al 202056 |
GECCO & NEAT, Germany |
High income | Retrospective | MSM | 2298 | January 2014 | Median 604 days (range 16–1353) | MSM 3–6 months | ≤6 |
| Johannesson et al 202057 |
TraP Hep C, Iceland |
High income | Prospective | PWID 85% | 597 | January 2016 | — | 3-monthly for active injectors, 6 monthly for others | ≤6 |
| Kaberg et al 202058 |
Needle Syringe Program, Stockholm, Sweden |
High income | Prospective | PWID | 124 | January 2018 | — | EOTc, SVR12 (at 3 months), then every 6 months | ≤6 |
| Kattakuzy et al 202059 |
ANCHOR, US |
High income | Prospective | PWID 100% | 82 | Median 96 weeks (24–96) | Post-SVR week 48, 72, 96 (= at 12, 18 and 24 months) | >6 | |
| Lens et al 202260 | Urban Harm Reduction Clinic, Spain | High income | Prospective | PWID | 168 | November 2018 | — | 6 monthly | ≤6 |
| Liu et al 202261 | RECUR study, Taiwan | Upper middle income | Prospective |
PLWHIV 92% MSM 5% PWID |
2016 | January 2005 | Median 3 years | 6 monthly | ≤6 |
| Marco et al 201962 |
Prisons, Catalonia, Spain |
High income | Retrospective |
Prisoners 100% PWID 74.1% |
602 | January 2002 | Mean 4.35 ± 2.7 years/reinfected participant | 12 months (or upon reincarceration) | >6 |
| Martinello et al 201663 |
ATAHC I, ATAHC II, DARE-C I and DARE-C II, Australia and New Zealand |
High income | Prospective |
HIV+ MSM 53% PWID (49% current, 69% ever used) |
120 | 2004 | 135py at risk | EOT, at post-treatment weeks 12, 24 and 48 (=at 3, 6 and 12 months) | ≤6 |
| Martinez-Rebollar et al 202164 | Hospital clinic, Spain | High income | Prospective |
PLWHIV 94% MSM |
290 | January 2010 | — | 6–24 monthly | ≤6 |
| Midgard et al 202165 |
Clinic Oslo, Norway |
High income | Prospective |
PWID |
488 | June 2013 | Median 6 months | 3 months | ≤6 |
| Minoyan et al 201866 |
HEPCO, Montreal, Canada |
High income | Prospective | PWID | 269 | January 2010 | — | 3-monthly in 2010, 6-monthly 2011–2017 | ≤6 |
| O'Sullivan et al 202067 |
ITTREAT, UK |
High income | Prospective |
PWUD PWID 92% |
109 achieved SVR; 76 retested | December 2013 | — | One off test 12 m post-SVR (48–60 weeks) | >6 |
| Schulkind et al 201968 |
Eradicate, Dundee, Scotland |
High income | Prospective | PWID | 105 | December 2012 | 42 months | At EOT, 3 m, 6 m then 18 m post-treatment = 3, 3 then 12 month intervals | >6 |
| Schutz et al 201823 | Drug treatment facility Vienna, Austria | High income | Prospective |
PWID 58% ongoing IDU |
40 | — | Mean 30.8 ± 13.4 months | Measured at SVR 12 and 24 (= at 3 months then 6 months) | ≤6 |
| Sylvestre et al 201769 |
OASIS (urban) methadone clinic, Oakland, California |
High income | Prospective | PWID—63% active IDU | 35 | — | — | One off test 12 months post-SVR | >6 |
| Valencia et al 201970 |
Harm Reduction Centre, Madrid, Spain |
High income | Prospective |
PWUD 73.8% IDU in last 6 month 52.5% IDU at last month |
160 | January 2016 | Median 0.6 years (IQR 0.3–1.3) | 3–6 months + when high-risk behaviours suspected | ≤6 |
| Wyles et al 201771 |
V-HICS, US |
High income | Prospective |
PLWHIV 56.6% PWID 44.9% prior IDU 0.49% current IDU |
205 | March 2015 | ≥52 weeks | 12 months | >6 |
| Young et al 201772 |
Canadian Co-infection Cohort, Canada |
High income | Prospective |
PWID and MSM 74% ever IDU 33% recent MSM activity |
257 | January 2003 | Median 1.5 years (IQR 0.6–3.2) | 6 months | ≤6 |
- a IQR Interquartile range.
- b SVR sustained virological response.
- c EOT end of treatment.
Twenty-seven studies reported reinfection among PWID, 9 among MSM and 3 among people in custodial settings (two studies reported on both PWID and MSM19, 20). No studies reporting reinfection among transgender people were identified. The interval between testing events for reinfection varied across included studies, ranging from as often as every 3 months to once 12 months post-sustained virological response (SVR) (Table 1).
Thirty-eight studies reported the number of HCV reinfections and the amount of person-time accrued, allowing for a calculation of a pooled HCV incidence rate estimate (Table S1). Three studies were identified that reported the proportion of participants diagnosed with a HCV reinfection, but reinfection incidence rate was not reported or could not be calculated from the available data. Gonzalez-Serna et al.21 tested for recently acquired HCV infection among HIV-infected participants in Spain and reported four cases of reinfection among 42 participants at risk (9.5%). Farley et al.22 measured reinfection post-SVR among Canadian correctional institutions and found 11 cases of reinfection among 132 participants. Schutz et al.23 reported two cases of HCV reinfection among 40 PWID participants between week 12 and 24 of follow-up post-SVR.
3.3 Pooled incidence estimates of HCV reinfection
The 38 studies included in the pooled HCV reinfection incidence estimate comprised 8931 participants at risk of reinfection (Table S1). Berenguer et al,19 and Chen et al20 included two study population arms (PWID and MSM) and HCV reinfection incidence rates were extracted for each arm and assigned to each key population meta-analysis separately. The pooled incidence estimate from all included studies was 4.13 per 100 py (95% confidence interval [CI]: 3.45–4.81) with HCV reinfection incidence ranging from 0.00 per 100 py to 31.00 per 100 py across studies. Heterogeneity was high among all included studies (I2 = 93.6%) (Figure 2).
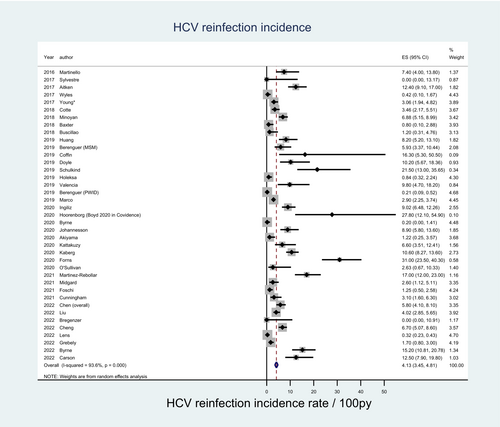
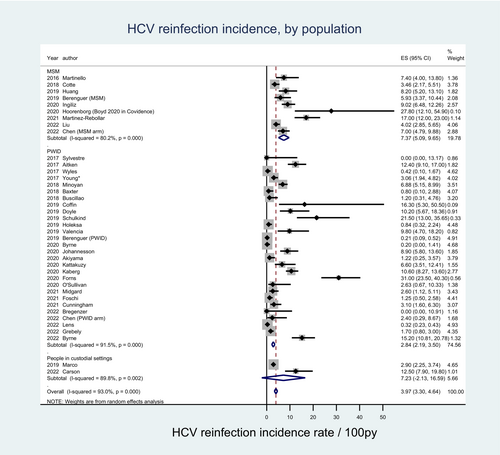
3.3.1 By key population groups
Among 27 studies comprising 4899 participants, where the primary study population were PWID, the pooled HCV reinfection incidence estimate was 2.84 per 100 py (95% CI: 2.19–3.50). Among the nine studies comprising 3269 participants whose primary population were MSM, the pooled incidence estimate was 7.37 per 100 py (95% CI: 5.09–9.65). Among the two studies comprising 763 participants who examined people in custodial settings, the pooled HCV reinfection incidence estimate was 7.23 per 100 py (95% CI: −2.13-16.59). Heterogeneity remained high across each subgroup estimate (Figure 3 and Table S2).
3.3.2 By testing interval
Twenty-three studies comprising 5058 participants were categorised as having testing intervals ≤6 months, with a pooled estimate of 4.26 per 100 py [95% CI: 2.86–5.65). Fifteen studies comprising 3873 participants were categorised as having testing intervals >6 months, with a pooled estimate of 5.19 per 100 py [95% CI: 3.92–6.46). High heterogeneity was observed across both testing interval groups (Figure 4 and Table S2).

3.3.3 By key population groups and testing interval
Among PWID, 17 studies comprising 3520 participants reported testing intervals of ≤6 months with HCV reinfection incidence rates ranging from 0.00 per 100 py to 21.50 per 100 py. Eleven studies comprising 1663 participants reported testing intervals >6 months, with incidence rates ranging from 0.00 per 100 py to 31.00 per 100 py. The pooled HCV reinfection incidence rate estimate was lower among PWID populations with testing intervals ≤6 months (2.97 per 100 py [95% CI: 1.55–4.39]) compared with those with testing intervals >6 months (3.96 per 100 py [95% CI: 2.64–5.29), though noting the presence of overlapping confidence intervals (Figure 5).
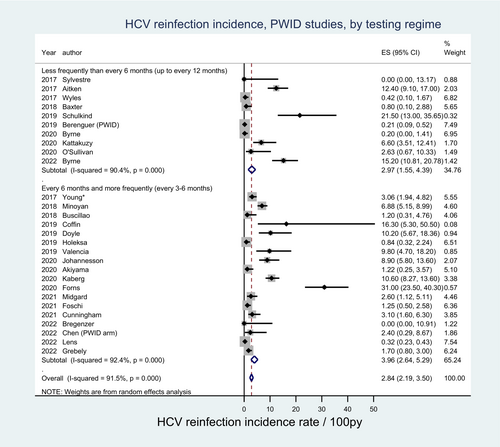
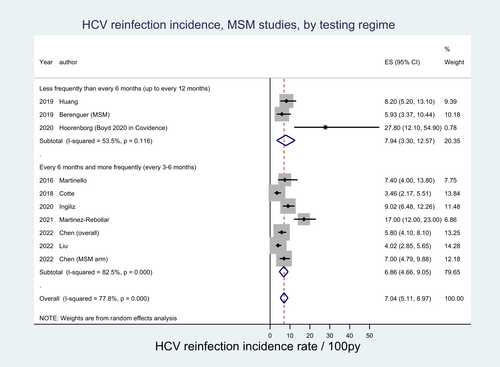
Among MSM, seven studies comprising 1945 participants reported a testing interval of ≤6 months with HCV reinfection rates ranging from 5.93 per 100 py to 27.80 per 100 py. Three studies comprising 1608 participants reported testing intervals >6 months, with HCV reinfection rates ranging from 3.46 per 100 py to 17.00 per 100 py. The pooled HCV reinfection incidence rate estimate among MSM populations was higher among studies with testing intervals ≤6 months (7.94 per 100 py [95% CI: 3.30–12.57]) compared with those with testing intervals >6 months (6.86 per 100 py [95% CI: 4.66–9.05]), though noting the presence of overlapping confidence intervals. Moderate-to-high heterogeneity was observed across all PWID and MSM groups (Figure 6). Among people in custodial settings, low study numbers limited the ability to compare testing frequencies.
3.4 Risk of bias
Thirty-one of the 41 observational studies were considered at low risk of bias (score ≥ 7) when graded using a modified Newcastle Ottawa Scale for cohort studies. The main biases identified were in determining the representativeness of the cohort, confirmation of the outcome and adequacy of follow up. The modified Newcastle Ottawa Scale for cohort studies and assessment scores are outlined in Appendices B and C, respectively.
4 DISCUSSION
In this systematic review of HCV reinfection intervals, we observed higher HCV reinfection incidence rates among studies including MSM compared with PWID. We detected no difference in HCV reinfection incidence based on retesting intervals. There has been interest globally in WHO guidelines development24 to determine optimal testing intervals for people at risk of HCV reinfection. A greater detection of HCV reinfection in studies with shorter testing intervals has been noted and explained previously, in part, due to less time for infections to spontaneously clear.25 Conversely, partial protective immunity from the primary infection may lead to a more rapid resolution of reinfection.26 It is also likely that higher incidence rates in studies which tested more frequently is biased by higher frequency testing protocols in studies of cohorts at greater risk of reinfection. This large meta-analysis has been able to explore both hypotheses and demonstrate no clear difference in very frequent (less than 6 months) or less frequent (more than 6 months) retesting.
Previous systematic reviews have estimated comparative pooled incidence rates of HCV reinfection among key populations and different HCV testing intervals. An earlier review found that rates of HCV reinfection were lower in studies of HCV mono-infected PWID, MSM and prisoners (1.91/100py) compared with HIV/HCV co-infected individuals (3.20/100py).27 Multiple reviews have reported a lower HCV reinfection incidence among individuals receiving opioid agonist therapy (OAT) (ranging from 0.55 to 1.4/100py) compared with those not receiving OAT.28, 29 Another meta-analysis among people living with HIV found that HCV reinfection was higher among MSM (5.89/100py) compared with people with recent injection drug use (5.49/100py).30 Another meta-analysis among HIV-infected MSM estimated an overall HCV reinfection rate of 5.27/100 py and found a higher reinfection rate among people with HCV test intervals of less than 6 months (7.59/100py) compared with those tested at greater than 6 month intervals (2.88/100 py).31 Among PWID populations, our findings are consistent with previously reported estimates in this group and are higher compared with studies examining HCV reinfection among MSM. A strength of this review is that all individuals at risk were compared using the same methodical approach allowing for better understanding of relative risks in these key populations.
Frequent testing is likely to increase case finding and be beneficial in reducing HCV disease burden for both the individual and community through early detection, treatment and cure as part of a TasP approach, and can be combined with other testing strategies, such as HIV and STI testing. This could potentially improve linkage to care and harm reduction support through increased engagement with healthcare services.15, 32 Although a person's risk of reinfection may decline over time, it is difficult to determine when in the post-SVR period any reinfection occurs, and thus if different testing intervals should be offered at different years. Routine HCV testing among people actively using drugs has been shown to be cost-effective in multiple settings, even when repeat testing leads to the need for repeat treatment, due to both the low cost of HCV testing and effective treatment.33 However, implementing testing for reinfection at regular intervals for all PWID may not be feasible in many settings. As testing for HCV reinfection relies on RNA or antigen testing, limited availability of these testing strategies may pose an additional challenge in certain settings. Further, in resource-constrained settings without universal healthcare or where systems that can deliver effective care post-diagnosis are not available, increased screening may not be cost-effective. Consideration should be given to higher short-term costs of tests, outpatient visits including healthcare personnel, the opportunity cost for time and resources diverted by healthcare staff, and if more re-infections are identified, the short-term costs of increased treatment. Furthermore, while frequent testing may diagnose individuals during the acute HCV phase, many DAAs are only approved for confirmed chronic HCV infection. Additionally, testing must be voluntary and offered alongside counselling to minimise the risk of stigma, discrimination and adverse psychological impacts.32, 34 While our analyses show no difference in the reinfection incidence rate based on retesting interval, recommendations for screening frequency should consider country-level or setting-specific contexts, including the availability of appropriate linkage to treatment following diagnosis.
There are important limitations to note when interpreting our findings. First, the purpose of our systematic review was to find RCTs and other comparative studies to determine the association between HCV testing frequency and HCV reinfection among key populations in the post-SVR period; however, only single-arm observational studies were identified. We have thus explored HCV reinfection between pooled estimates of observational studies, limiting our ability to determine the effectiveness of different testing regimes on HCV detection. Second, only studies that tested at discrete time intervals were included, and as such results were more representative of studies from high-income countries. Studies where clinicians self-selected when to offer testing were not included to reduce the influence of higher risk individuals being tested more often. Third, only nine studies were included among MSM and two among people in custodial settings, limiting our ability to discern meaningful differences between testing frequencies for these key populations. Fourth, our analysis could not account for confounding factors, such as age, gender and socioeconomic status since it was inconsistently reported within studies. Fifth, the studies included in this review contain significant heterogeneity among population characteristics, where some studies included participants with life-time drug use, those with only recent drug use or injecting drug use or those on opioid substitution therapy or other risk reduction measures. Due to this heterogeneity, along with incomplete reporting by study authors, we were unable to disaggregate the PWID sub-population by recent and ever injecting drug use, limiting the ability to confidently detect differences between these groups. This heterogeneity has also been observed in previous systematic reviews including the ones described above. Additionally, the inclusion of both recent and past injecting exposure within the PWID cohorts, and the fact that testing frequency within study protocols may have been influenced by the risk profile of included participants (i.e. less frequent testing for those without recent injecting exposure) is likely to be a significant factor altering estimates of incidence of reinfection among this key population. Sixth, our review did not find any studies examining HCV reinfection among transgender people. While it is possible that transgender people were included in our studies, often as part of MSM cohorts, this finding is most likely reflective of deficiencies in the collection and reporting of gender identity in health records and research. The adoption of a gender lens to HCV care and research to explore the impacts of gender disparities on HCV elimination is required.35 Finally, as nearly all included studies were from high-income country settings, any consideration of HCV testing frequency recommendations should be applied to high-income settings only due to differences in HCV risk context including local drivers of HCV transmission and availability of resources. This highlights the crucial need for more context-specific HCV elimination research from low- and middle-income countries.
This systematic review advances our understanding of how different testing intervals influence HCV detection among PWID, MSM, and people in custodial settings. Furthermore, this review updates the HCV reinfection incidence estimates among these key populations. Our findings have highlighted the absence of high-quality trial or cohort data to make direct comparisons on testing frequency, and suggest that future longitudinal studies comparing annual testing with more frequent testing (i.e. 3–6 monthly) among key populations are needed. Our findings have direct implications for clinical practice and have contributed to WHO global testing recommendations for key populations, where people at ongoing risk and a history of previous HCV infection may be offered 3–6 monthly HCV testing where appropriate and available.24 Increasing voluntary testing frequency coupled with offers of HCV treatment among people at ongoing risk could have significant individual and population level benefits, enabling further progress towards global HCV elimination.
AUTHOR CONTRIBUTIONS
Joseph S. Doyle is the guarantor of this article. Authors Joseph S. Doyle, Margaret E. Hellard, Mark A. Stoové, Ned H. Latham, Rachel Baggaley, Virginia MacDonald, Annette Verster, Nandi Siegfried, Stephanie C. Munari, Michael W. Traeger, Virginia MacDonald, Ned H. Latham and Lakshmi Manoharan contributed to the conceptualisation and design of the research study. Nandi Siegfried provided methodological expertise. Brian Conway, Marina Klein and Julie Bruneau contributed to the acquisition and interpretation of data. Stephanie C. Munari, Michael W. Traeger and Vinay Menon performed the search, data collection and analysis. Stephanie C. Munari, Michael W. Traeger and Joseph S. Doyle prepared the original draft manuscript. All authors were involved in reviewing and revising the manuscript and have approved the final version for publication.
ACKNOWLEDGEMENTS
Co-authors from the World Health Organisation Global Hepatitis Programme were involved in the conceptualisation and design of the research study, the interpretation of data, reviewing and revising the manuscript and in the decision to submit the manuscript for publication.
FUNDING INFORMATION
This work was supported by the World Health Organisation's Global HIV, Hepatitis and Sexually Transmitted Infections Programmes who commissioned and funded the project. Copyright in the original work on which this Article is based belongs to WHO. The authors alone are responsible for the views expressed in this Article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated. Burnet Institute acknowledges support from the Victorian Government Operational and Infrastructure Fund. MWT, JSD, MEH and MAS acknowledge fellowship support from the National Health and Medical Research Council.
CONFLICT OF INTEREST STATEMENT
MWT has received speaker's fees and investigator-initiated research funding from Gilead Sciences. NS provides consultancy to act as a methodologist for the WHO guidelines related to this manuscript including contracts and consulting fees. JSD, MEH and MAS's institution has received funding for research and speaking from Gilead Sciences and AbbVie. MAS reports unrelated consultancy fees and advisory board participation for Gilead Sciences and AbbVie. JB receives grants or contracts from Canadian Institutes on Health Research, Gilead Sciences, National Institute on Drug Abuse (USA) and AbbVie and reports advisory board participation for Gilead Sciences and AbbVie. All other authors declare no potential conflicts of interest.
APPENDIX A: Search strategies
- exp Hepatitis C/ or exp Hepatitis C, Chronic/
- (hepatitis c or hepatitis c virus or hcv).mp
- 1 or 2
- (test* or screen*).mp
- (antigen or RNA).mp
- 4 or 5
- re?infection).mp
- exp Incidence/ or inciden*.mp
- 7 or 8
- 3 and 6 and 9
- limit 11 to (yr=”2014-current”)
Yield = 4376
- exp Hepatitis C/ or exp Hepatitis C, Chronic/
- (hepatitis c or hepatitis c virus or hcv).mp
- 1 or 2
- (test* or screen*).mp
- (antigen or RNA).mp
- 4 or 5
- re?infection).mp
- exp Incidence/ or inciden*.mp
- 7 or 8
- 3 and 6 and 9
- limit 11 to (yr=”2014-current”)
Yield = 1011
Web of Science
TS = (hepatitis C or HCV)
TS = (test* or screen*)
TS = (antigen or RNA or ribo$nucleic)
2 or 3
TS = (re$infection or inciden*)
1 and 4 and 5.
Timespan: 2014–2021 to the above terms.
Yield = 1677.
APPENDIX B: Quality appraisal checklist
Modified Newcastle-Ottawa quality assessment scale for cohort studies.
| Selection |
|
|
|
|
|
|
|
| Outcome |
|
|
|
|
|
|
APPENDIX C: Risk of Bias scores for included observational studies.
| Study | 1) Representativeness of the cohort | 2) Clear definition of study population provided | 3) Ascertainment of testing frequency interval in study population | 4) Demonstration that outcome of interest was not present at start of study | 5) Assessment of outcome | 6) Confirmation of outcome | 7) Was follow-up long enough for outcomes to occur | 8) Adequacy of follow-up of cohort | Total score (/9) |
|---|---|---|---|---|---|---|---|---|---|
| Aitken et al, 2017 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Akiyama et al, 2020 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 9 |
| Baxter et al, 2018 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Berenguer et al, 2019 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Bouscillao et al, 2018 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Bregenzer et al, 2022 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 5 |
| Byrne et al, 2020 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Byrne et al, 2022 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Carson et al, 2022 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 9 |
| Chen et al, 2022 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 8 |
| Cheng et al, 2022 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Coffin et al, 2019 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 9 |
| Cotte et al, 2018 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Cunningham et al, 2021 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 9 |
| Doyle et al, 2019 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| Farley et al, 2018 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Forns et al, 2020 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 3 |
| Foschi et al, 2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Grebely et al, 2022 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 9 |
| Gonzalez-Serna et al 2020 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Holeksa et al, 2019 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 9 |
| Hoorenborg et al, 2020 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Huang et al, 2019 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Ingiliz et al, 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Johannesson et al, 2020 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 8 |
| Kaberg et al, 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Kattakuzy et al, 2020 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 5 |
| Lens et al, 2022 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 8 |
| Liu et al, 2022 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Marco et al, 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Martinello et al, 2016 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 9 |
| Martinez-Rebollar et al, 2021 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Midgard et al, 2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Minoyan et al, 2018 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| O'Sullivan et al, 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Schulkind et al, 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Schutz et al, 2018 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Sylvestre et al, 2017 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 5 |
| Valencia et al, 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Wyles et al, 2017 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Young* et al, 2017 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supplementary material of this article.