Comparison of efficacy of arthroscopic lavage plus administration of corticosteroids, arthroscopic lavage plus administration of placebo, and joint aspiration plus administration of corticosteroids in arthritis of the knee: A randomized controlled trial
Abstract
Objective
To compare the efficacy of arthroscopic lavage plus administration of corticosteroids (ALC), arthroscopic lavage plus administration of placebo (ALP), and joint aspiration plus administration of corticosteroids (JAC) in knee arthritis, and to evaluate whether clinical or histologic characteristics determine outcome.
Methods
Patients with knee arthritis (not due to gout, osteoarthritis, or septic arthritis) were randomized over 3 treatment arms: ALC, ALP, and JAC. The primary end point was event-free survival, with events defined as 1) recurrence or persistence of symptomatic knee swelling necessitating local re-treatment, or 2) nonimprovement of the knee joint score. Synovial tissue specimens were collected and analyzed histologically to identify predictive factors of responsiveness.
Results
A total of 78 patients were enrolled; 3 patients did not receive their allocated therapy and 3 were lost to followup. The median time until recurrence was 9.6 months after ALC, 3.0 months after JAC, and 1.0 month after ALP, corresponding to a relative risk (RR) of arthritis recurrence of 2.2 for JAC (95% confidence interval [95% CI] 1.2–4.2, P = 0.02) and 4.7 for ALP (95% CI 2.3–9.4, P < 0.0001) compared with ALC. A high versus low synovial extent of fibrosis conferred an RR for recurrence of 5.7 (95% CI 1.6–20.5, P < 0.01) after ALC.
Conclusion
Arthroscopic lavage plus administration of corticosteroids was more effective than arthroscopic lavage plus administration of placebo or joint aspiration plus injection of corticosteroids. The absence of fibrosis was a histologic predictor of a beneficial response.
INTRODUCTION
Intraarticular corticosteroid injection is a valuable adjunct local therapy in patients with rheumatic diseases and persisting or recurring arthritis of a joint despite treatment with antirheumatic drugs. Corticosteroid injections can lead to a quick relief of symptoms, are well tolerated, and are considered generally safe if no more than 3–4 injections per year per joint are administered (1). Several studies have examined factors that prolong the therapeutic effect of corticosteroids. These include postinjection rest, maximal synovial fluid aspiration, and lavage or washout of the joint (2-4). The therapeutic effect of joint lavage is attributed to optimal removal of inflammatory cells, cytokines, and degradation products of the inflamed synovium, facilitating the antiinflammatory effects of intraarticular corticosteroid administration (5). Nevertheless, studies comparing the therapeutic benefit of arthroscopic lavage plus corticosteroid administration as opposed to lavage or corticosteroid administration alone have been inconclusive, in part due to differences in therapeutic interventions and (uncontrolled) study designs (4, 6-8). In a retrospective study (9), we found a difference in duration of symptom relief in favor of arthroscopic lavage (with or without corticosteroid administration) compared with joint aspiration plus corticosteroid administration. Because of the uncontrolled design of this study, we set up a prospective randomized trial to study the efficacy of arthroscopic lavage plus administration of corticosteroids, arthroscopic lavage plus administration of placebo, and joint aspiration plus administration of corticosteroids in patients with arthritis of the knee. Synovial tissue specimens were collected from all patients at baseline to examine predictive factors of responsiveness by synovial infiltrate analysis.
PATIENTS AND METHODS
Patients.
Patients were recruited from the outpatient clinic of the Department of Rheumatology of the Leiden University Medical Center (LUMC) between July 2002 and December 2003. Eligibility criteria included a minimum age of 18 years and arthritis of the knee not due to gout, osteoarthritis, or infection. Exclusion criteria were hemorrhagic disease, oral prednisone >10 mg/day, recent (<6 weeks) change of disease-modifying antirheumatic drugs (DMARDs), recent joint injection (any joint), and hypersensitivity to methylprednisolone or local anesthetics. Patients were allowed to use acetaminophen, nonsteroidal antiinflammatory drugs, other analgesics, and DMARDs, and any changes in dose regimen were recorded. The protocol was approved by the LUMC Ethics Committee and written informed consent was obtained from all patients.
Interventions.
Patients were randomized to 1 of 3 interventions based on a computer-generated list prepared by the statistician (JKS). Allocation of the interventions was based on the chronological order of registration and was performed by an independent physician unaware of patients' identities. Patients and the research nurses and treating rheumatologists who served as assessors (see below) were not informed about the results of randomization. Each procedure was performed in an outpatient setting under aseptic conditions in a designated room with a setup aimed at maximal blinding of the patient (dimmed lights, supine position). The 3 interventions were arthroscopic lavage with administration of corticosteroids (ALC), arthroscopic lavage with administration of placebo (ALP), and joint aspiration with administration of corticosteroids (JAC). All interventions were performed by one person (MO) and included the following consecutive steps: 1) maximal aspiration of synovial fluid from the suprapatellar pouch, 2) intraarticular injection of 30 ml of lidocaine 0.5% through the aspiration needle, and 3) additional infiltration of the skin and joint capsule with 10 ml of lidocaine 1%. The subsequent steps of each intervention are described below.
Arthroscopic lavage plus administration of corticosteroids.
The skin inferolateral to the patella was also injected with 10 ml of lidocaine 1%. Two skin incisions were made for the introduction of 2 trocars into the joint cavity: 1 inferior for introduction of the camera and administration of saline, and 1 superior for drainage of the irrigation fluid and introduction of the biopsy forceps. A total of 1,000 ml of saline was flushed through the joint. During lavage, 15–20 synovial biopsy samples were obtained with 2.0-mm forceps (Storz, Nieuwegein, The Netherlands). After lavage, a mixture of methylprednisolone (80 mg in 2 ml) and bupivacaine (30 mg in 6 ml) was injected through the inferior trocar.
Arthroscopic lavage plus administration of placebo.
The ALP intervention was identical to ALC, but only bupivacaine (30 mg in 8 ml; referred to as placebo) was administered.
Joint aspiration plus administration of corticosteroids.
A single skin incision was made and 1 trocar was introduced. After synovial biopsy samples (15–20 specimens) were obtained, a mixture of methylprednisolone (80 mg in 2 ml) and bupivacaine (30 mg in 6 ml) was injected into the joint.
At the end of each procedure, skin incisions were closed with steristrips and patients were advised to limit physical activities involving the knee for 48 hours. Patients were seen every 3 months by their rheumatologist or more frequently when necessary. Study visits took place at 0, 1, 3, 6, and 9 months.
Outcome measures and followup.
The primary outcome measure was event-free survival defined as the time in months after treatment until local re-treatment (joint aspiration or injection, arthroscopy, or [radio]synovectomy) because of recurrence or persistence of arthritis of the knee as determined by the treating rheumatologist, or nonimprovement of knee joint score assessed by trained research nurses. The knee score (range 0–7) encompasses knee tenderness as scored from 0 to 3 (0 = no tenderness, 1 = tenderness when asked, 2 = tenderness on pressure, and 3 = tenderness and wincing) (10), knee swelling as scored from 0 to 3 (0 = no swelling, 1 = little swelling, 2 = moderate swelling, and 3 = abundant swelling) (11), and a patient visual analog scale (VAS; range 0–100 where 0 = no pain and 100 = maximal pain) for knee pain as scored from 0 to 1 (VAS score divided by 100). Secondary outcomes were adverse events, serologic, and immunohistochemical parameters. Furthermore, tender and swollen 28-joint scores were obtained in all patients. In patients with rheumatoid arthritis (RA) (12), a Disease Activity Score in 28 joints was calculated, which is a composite score for joint pain and tenderness in 28 joints, erythrocyte sedimentation rate (ESR), and a VAS for general well-being (range 0–100) (13). Laboratory investigations included IgM rheumatoid factor, full blood count, ESR, C-reactive protein (CRP) level, and renal and liver function tests. At baseline, standard weight-bearing radiographs of both knees were made. Radiographs were scored for severity of osteoarthritis on the Kellgren and Lawrence (K/L) scale (14) for joint space narrowing, osteophytes, and sclerosis. This score was divided into 2 categories depending on the absence (K/L score 0–1) or presence (K/L score 2–4) of osteoarthritis.
Synovial tissue analysis.
Synovial biopsy samples were obtained from all patients in a standardized manner (15). Synovial specimens were embedded in paraffin, sectioned at 3 μm, and stained with hematoxylin and eosin. Coded sections were scored for the following histologic features: mean synovial lining layer thickness from 6 random places; mean number of infiltrating lymphocytes, plasma cells, and neutrophils from 3 random high-power fields (× 400); vascularity expressed as the number of blood vessels with detectable endothelial cells in 3 high-power fields (× 200); and presence of fibrosis and fibrin (range 0–4, where 0 = absent and 4 = abundantly present).
Statistical analysis.
The determination of the sample size was based on detectable differences in the primary end point, event-free survival. A difference in event-free survival of 25% after 9 months (9) between any 2 of the 3 interventions was calculated to have a 90% power with a sample size of 26 patients per treatment arm (α = 0.05) and a dropout rate of 20%. The cumulative incidence of events for treatment groups was determined by constructing Kaplan-Meier survival curves. A possible difference in recurrence rate between the treatments was examined through a variant of the Cox proportional hazards regression model that allows for the participation of patients in all treatments by robust variance estimates (16). Dichotomous variables were created for clinical and synovial characteristics. The Cox model was used to evaluate a possible confounding effect of dichotomized characteristics on the recurrence rate. Furthermore, we assessed whether the recurrence rate after treatments differed between categories of clinical parameters. The Cox model produces a regression coefficient for each covariate that can be interpreted as a relative risk (RR) when taken exponentially. Other statistical analyses were performed using Student's t-test, Wilcoxon's signed rank test, analysis of variance, and chi-square test where appropriate. All statistical analyses were performed with the statistical software package STATA 6.0 (Stata, College Station, TX). P values less than 0.05 were considered statistically significant.
RESULTS
Patient characteristics.
A total of 78 patients with arthritis of the knee were enrolled in the study. Of these patients, 75 were analyzed: 51 with RA, 15 with spondylarthropathy, and 9 with undifferentiated arthritis. Three patients allocated to ALP were excluded from analysis because of a treatment violation (the patients insisted on treatment with ALC and were treated accordingly) and 3 patients (1 in each group) were lost to followup. A flow diagram of the study is shown in Figure 1. The treatment groups were comparable at baseline regarding demographic, clinical, and synovial characteristics except for synovial lining layer thickness and neutrophils, which were statistically lower in the JAC group (P = 0.005 and P = 0.04, respectively) (Table 1).
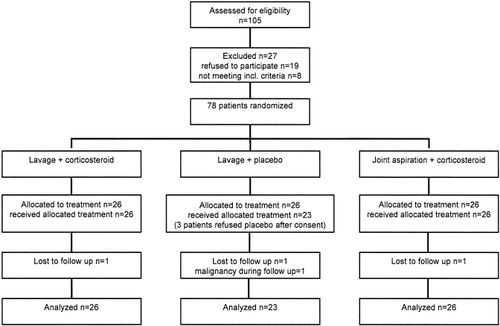
Trial profile. Incl = inclusion.
| ALC (n = 26) | ALP (n = 23) | JAC (n = 26) | P† | |
|---|---|---|---|---|
| Demographic and disease characteristics | ||||
| Age, years | 51.7 ± 14.0 | 59.6 ± 16.0 | 50.7 ± 17.3 | 0.11 |
| Sex, male/female | 12/14 | 12/11 | 10/16 | 0.64 |
| Diagnosis, no. | ||||
| RA (no. rheumatoid factor positive) | 16 (14) | 18 (9) | 17 (12) | 0.41 |
| Spondylarthropathy | 6 | 2 | 7 | |
| Undifferentiated arthritis | 4 | 3 | 2 | |
| Disease duration, years | 5.7 ± 5.4 | 7.4 ± 6.9 | 9.5 ± 8.9 | 0.17 |
| No. previous DMARDs | 2.0 ± 1.8 | 2.4 ± 2.3 | 1.9 ± 1.9 | 0.74 |
| ESR, mm/hour | 33.8 ± 32.0 | 35.9 ± 30.3 | 27.6 ± 22.8 | 0.56 |
| CRP, mg/liter | 38.9 ± 38.2 | 42.8 ± 55.9 | 20.3 ± 21.3 | 0.12 |
| DAS28‡ | 3.97 ± 1.1 | 3.82 ± 1.3 | 4.15 ± 1.2 | 0.62 |
| No. tender joints (0–28) | 3.1 ± 2.9 | 3.6 ± 5.4 | 4.0 ± 4.2 | 0.35 |
| No. swollen joints (0–28) | 2.4 ± 1.7 | 2.4 ± 1.2 | 2.8 ± 1.8 | 0.35 |
| VAS general well-being, mm | 38.7 ± 22.6 | 27.9 ± 21.2 | 45.6 ± 29.2 | 0.05 |
| Knee characteristics | ||||
| Knee score (0–7) | 3.5 ± 1.3 | 3.8 ± 1.1 | 3.9 ± 1.6 | 0.51 |
| No. previous knee injections | 2.9 ± 4.0 | 3.7 ± 3.8 | 3.4 ± 5.1 | 0.80 |
| SF volume, ml | 36.3 ± 37.6 | 31.4 ± 23.8 | 50.2 ± 102.1 | 0.58 |
| SF leukocyte count, × 109/liter | 8.0 ± 7.6 | 13.4 ± 13.1 | 8.2 ± 9.3 | 0.16 |
| K/L score 0–1, no. of points | 21 | 14 | 21 | 0.13 |
| K/L score 2–4, no. of points | 4 | 9 | 5 | |
| Histologic features of synovium | ||||
| Synovial lining thickness | 3.7 ± 1.3 | 3.1 ± 1.0 | 2.5 ± 0.8 | 0.005 |
| Lymphocytes (no./hpf) | 43.9 ± 43.2 | 45.3 ± 43.7 | 36.2 ± 44.9 | 0.79 |
| Plasma cells (no./hpf) | 12.5 ± 16.0 | 8.8 ± 16.2 | 11.0 ± 12.4 | 0.75 |
| Neutrophils (no./hpf) | 7.6 ± 10.0 | 12.4 ± 19.8 | 1.2 ± 2.27 | 0.04 |
| Vascularity (no./hpf) | 21.9 ± 8.3 | 23.0 ± 11.0 | 18.9 ± 8.6 | 0.38 |
| Fibrin score (0–4) | 1.0 ± 1.4 | 2.0 ± 1.5 | 1.1 ± 1.3 | 0.07 |
| Fibrosis score (0–4) | 1.5 ± 1.0 | 1.7 ± 1.4 | 2.1 ± 1.3 | 0.35 |
- * Values are the mean ± SD unless otherwise indicated. ALC = arthroscopic lavage plus corticosteroids; ALP = arthroscopic lavage plus placebo; JAC = joint aspiration plus corticosteroids; RA = rheumatoid arthritis; DMARDs = disease-modifying antirheumatic drugs; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; DAS28 = Disease Activity Score in 28 joints; VAS = visual analog scale; SF = synovial fluid; K/L = Kellgren/Lawrence; hpf = high-power field.
- † All factors were tested with analysis of variance except diagnosis and K/L score, which were tested with chi-square tests.
- ‡ Only assessed in patients with RA.
Clinical efficacy.
ALC was the most effective therapy, followed by JAC and ALP (Figure 2). The median event-free survival time was 9.6 months after ALC, 3.0 months after JAC, and 1.0 month after ALP. The estimated RR of an event during the 9 months following intervention was 2.2 (95% confidence interval [95% CI] 1.2–4.2, P = 0.02) for JAC and 4.7 (95% CI 2.3–9.4, P < 0.0001) for ALP compared with ALC. These results were independent of the number of previous joint injections (data not shown). The RR between ALP and JAC was 2.0 (95% CI 1.1–3.8, P = 0.01) in favor of joint aspiration. Twenty-two patients (13 after ALC, 8 after JAC, and 1 after ALP; P < 0.01) completed the 9 months without recurrence of arthritis. Thirty-seven patients (8 after ALC, 13 after JAC, and 16 after ALP; P = 0.26) reached the primary end point because of re-treatment and 15 patients (5 in each group) reached the end point because of nonimprovement of the knee score. Thirteen of 22 patients who did not experience an event had no DMARD change during followup (9 after ALC, 3 after JAC, and 1 after ALP; P = 0.02). The number of patients with a change of DMARD therapy (regardless of recurrence of arthritis) per month followup was skewed between interventions: 0.04 for ALC versus 0.06 for ALP and JAC (P = 0.36). There was no difference in disease activity or ESR between the groups during the followup period (P = 0.73 and P = 0.12, respectively). Eighteen patients had >1 local reintervention of the knee within the year following treatment.
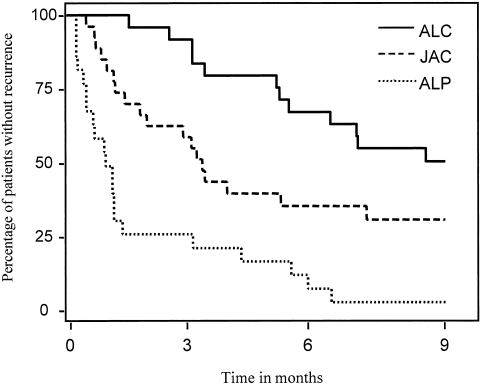
Kaplan-Meier survival curves for event-free survival after arthroscopic lavage plus administration of corticosteroids (ALC), arthroscopic lavage plus administration of placebo (ALP), and joint aspiration plus injection of corticosteroids (JAC).
The knee score showed a greater decrease after ALC compared with both of the other interventions (Figure 3). The difference in decrease was significant for ALC compared with ALP after 1 month (1.93 versus 0.08; P < 0.01) and 3 months (1.63 versus 0.86; P < 0.04), and for JAC compared with ALP at 1 month (1.77 versus 0.08; P < 0.01). After 6 months, too many patients had had a reintervention to make meaningful comparisons (6 in ALC, 13 in JAC, and 13 in ALP).
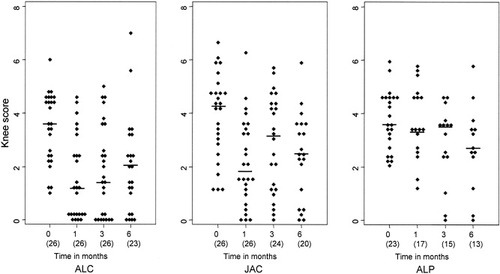
Knee scores in patients who completed followup without re-treatment. Baseline, 1-month, 3-month, and 6-month data of patients treated with arthroscopic lavage plus administration of corticosteroids (ALC), arthroscopic lavage plus administration of placebo (ALP), and joint aspiration plus injection of corticosteroids (JAC) are shown. The numbers in parentheses are the number of patients without reintervention.
Clinical and synovial factors and treatment effect.
Using the Cox proportional hazards regression analysis, no clinical or synovial factors were found to have a confounding effect on the differences in responses between treatment groups. For example, although the number of patients with a diagnosis of spondylarthropathy was lower in the ALP group, this was not associated with a significant difference in outcome (P = 0.35). Similarly, the difference in synovial lining thickness in biopsy samples obtained at baseline did not have a significant impact on outcome either (P = 0.22). Clinical and synovial factors were also studied as predictors for treatment outcome and were dichotomized to calculate an RR within one treatment arm between both subgroups of a variable. In continuous variables, the median served as the arbitrary cutoff point for dichotomization. A greater degree of fibrosis compared with a lesser degree in the synovial tissue (≥2 versus <2, 2 representing the median) conferred an RR of 5.7 (95% CI 1.6–20.5, P < 0.01) after ALC (Figure 4). The effect of fibrosis on outcome was confirmed when the knee score was taken as a parameter. ALC resulted in a more pronounced decrease in knee score in patients with a low versus high extent of fibrosis: 2.5 versus 1.5 at 1 month (P = 0.02), 2.5 versus 1.0 at 3 months (P < 0.01), and 1.8 versus 1.5 at 6 months (P = 0.27). No differences were detectable in the other treatment arms. None of the other histologic features had a statistically significant impact on outcome (data not shown). Patients with >1 previous injection had an RR of 2.4 (95% CI 1.5–6.3, P = 0.006), and patients with 0–1 injections had an RR of 2.0 (95% CI 1.2–3.2, P = 0.04) for recurrence within 9 months after JAC when compared with ALC.

Kaplan-Meier curves for event-free survival after arthroscopic lavage plus administration of corticosteroids (ALC) in relation to the extent of fibrosis in synovium at baseline.
Combination of variables.
Correlation analysis demonstrated an incomplete overlap of absence of fibrosis and lymphocyte infiltration in a proportion of the patients. The presence of fibrosis showed an inverse relationship with the number of infiltrating lymphocytes in all 3 groups (Spearman's ρ = −0.43, P = 0.01). ESR at baseline correlated inversely with synovial fibrosis in the lavage with corticosteroid group (ρ = 0.55, P = 0.016). A correlation was found between the leukocyte count (granulocytes) in synovial fluid and CRP levels (ρ = 0.63, P < 0.001) and between synovial fluid leukocyte count and knee swelling (ρ = 0.25, P = 0.045).
Adverse events.
No treatment-related infections occurred during the study. One patient (ALC) died of sudden cardiac arrest 6 months after the intervention. One patient (ALP) was diagnosed with lymphoma shortly after treatment. One patient (JAC) developed hemarthrosis, 1 patient (ALP) experienced prolonged skin bleeding, and 3 patients (1 in each group) experienced transient local symptoms around the superior skin incision. All intervention-related side effects resolved spontaneously.
DISCUSSION
The present study compared 3 local therapies for arthritis of the knee. We opted to perform a practice-based study with results that are applicable in usual care. As a corollary, we included patients with a variety of nonseptic arthritides and used a pragmatic outcome measure based on the need for further local intervention. We also used a composite knee score as a separate secondary measure of local arthritis activity (11). ALC markedly prolonged the median time until recurrence of symptomatic arthritis when compared with ALP or JAC. Both interventions with corticosteroid administration resulted in a significantly greater improvement of knee score after 1 month when compared with lavage alone, underlining the beneficial effect of corticosteroids. The difference in effect between the 3 interventions was not confounded by differences in clinical characteristics such as disease duration, underlying diagnosis, systemic disease activity, or knee characteristics such as leukocyte count in the synovial fluid or concomitant osteoarthritis in which lavage plus steroid injection is ineffective (17). These data suggest that the initial antiinflammatory effect of corticosteroid administration can be prolonged with lavage due to a more complete removal of degradation products (e.g., fibrin) (18).
The mean number of intraarticular injections per patient prior to this study (3.3 overall) indicates that most patients in our study experienced refractory arthritis of the knee. ALC appears to be a good alternative as local treatment for such patients, although its superior effect was also present in patients who had received 0–1 previous injection.
Some confounding factors were present. Blinding of patients and rheumatologists was incomplete because a sham second skin incision in patients randomized to JAC was not performed. This may have resulted in overestimation of the clinical benefits of ALC and ALP, although the poor response in the latter group indicates that the placebo effect of the arthroscopic procedure and lavage was negligible. In contrast, the effect of JAC as a surrogate for routine joint aspiration and corticosteroid injection may be an overestimate of the true effect of the latter, because data from controlled trials have indicated that 10% of corticosteroids are deposited extraarticularly (19, 20). Extraarticular deposition of corticosteroids was unlikely involved in our study because synovial tissue specimens were obtained successfully in all patients through use of a trocar instead of a needle.
Previous studies have not been conclusive regarding the therapeutic value of lavage in patients with arthritis of the knee, in part due to differences in study design and small numbers of patients (4, 6, 8). One randomized study failed to demonstrate superiority of arthroscopic lavage and corticosteroid administration versus joint aspiration and corticosteroid administration alone (8). In the present study, a larger amount of saline and 80 mg of methylprednisolone were used compared with 60 mg of triamcinolone acetonide used in the previous study (8). The advantage of a more extensive washout has previously been shown (21). Furthermore, the followup in our study was 9 months compared with only 3 months in other studies on arthroscopic lavage (4, 8). Because many of our patients had refractory arthritis of the knee, a sustained clinical effect is of great value. In contrast to previous reports, lavage alone was not effective in our study. The results from the present study are in keeping with our previous study (9), both showing better efficacy of lavage (without corticosteroids, taken as one group) over aspiration (with corticosteroids), although differences in design preclude detailed comparisons (e.g., prospective versus retrospective, blinding versus nonblinding).
Our study is the first to demonstrate the absence of fibrosis in synovium to be an independent predictive factor of responsiveness to lavage with corticosteroid as local treatment for arthritis. Whether extensive synovial fibrosis is a feature of a different form of joint inflammation or reflects an advanced stage of chronic inflammation remains to be determined. A recent gene expression analysis of synovial tissue specimens from patients with RA revealed that 2 types of synovitis can be recognized, corresponding to a high versus low inflammatory subset (22). The latter predominantly expressed genes involved in repair and fibrosis. One study demonstrated a better clinical outcome after high-dose immunosuppressive therapy in patients with refractory RA and a lymphocytic synovial infiltrate (23), whereas fibrosis was used as a synovial biomarker in RA and osteoarthritis in 2 other studies (24, 25). Further studies are needed to determine the predictive value of histologic features, and fibrosis in particular, in responsiveness to therapy.
In conclusion, ALC offers superior therapeutic benefit in patients with arthritis of the knee in comparison with arthroscopic lavage alone or JAC. A strikingly better response was found in patients with less fibrotic synovial tissue. As an outpatient treatment, ALC is well tolerated, safe, and effective and can be considered a valuable alternative for the local treatment of patients with arthritis of the knee.




