Giant cell arteritis: Involvement of intracranial arteries
Introduction
Cerebrovascular ischemic events have been reported in 3–4% of patients with giant cell arteritis (GCA) (1, 2). Clinical and pathologic findings suggest that these ischemic events are due to involvement of extradural vertebral and carotid arteries with high-grade stenosis or occlusion rather than intracranial vasculitis (3-10).
Several previous reports have described patients with cerebral vasculitis who were also considered to have GCA. However, most of these patients probably represent cases of primary central nervous system vasculitis (PCNSV), a different disease with similar histopathologic findings (11, 12). To determine the occurrence and frequency of central nervous system (CNS) vasculitis in GCA, we reviewed the medical records of all patients with a diagnosis of CNS vasculitis or angiitis at the Mayo Clinic (Rochester, MN) over a 17-year period and identified those with both biopsy-proven GCA and pathologic and/or angiographic evidence of intracranial vasculitis.
Patients and Methods
Using the Mayo Clinic medical records linkage system, a list of all patients with a medical diagnosis of CNS vasculitis or angiitis between January 1, 1987 and December 31, 2003 was provided. All 463 identified medical records were reviewed. The systemic vasculitides and connective tissue diseases that were diagnosed are shown in Table 1. Three patients presented with biopsy-proven GCA. However, in 1 of the 3 patients, high-grade stenosis of the internal carotid and vertebral arteries was extradural just proximal to the dural entry point. Therefore the patient was not considered to be affected by intracranial GCA (5). We describe the other 2 patients with GCA who presented pathologic and/or angiographic evidence of intracranial vasculitis.
| Condition | No. patients |
|---|---|
| Isolated CNS vasculitis | 73 |
| ANCA-associated vasculitis | 13 |
| Behçet's disease | 8 |
| Giant cell arteritis | 3 |
| Undefined vasculitis | 3 |
| Systemic lupus erythematosus | 9 |
| Sjögren's syndrome | 2 |
| Rheumatoid arthritis | 2 |
| Undefined connective tissue disease | 1 |
- * CNS = central nervous system; ANCA = antineutrophil cytoplasmic antibody.
Case 1.
A 67-year-old woman presented with new-onset headache, jaw claudication, left-sided visual field cut, and pain with morning stiffness (>1 hour) in the shoulders, arms, and hips. A diagnosis of polymyalgia rheumatica (PMR) and GCA was suspected. Erythrocyte sedimentation rate (ESR) was 92 mm/hour. Temporal artery biopsy results confirmed the diagnosis of GCA. Prednisone 100 mg/day was started with resolution of the patient's symptoms within a few days. The prednisone dose was progressively reduced. Twelve months later when the patient was taking prednisone 5 mg/day, symptoms of PMR recurred and prednisone was increased to 20 mg/day. One year later, while taking prednisone 10 mg/day, the patient developed transitory ischemic attacks that consisted of 5-minute intermittent spells of left facial and left upper-extremity tingling. There was also a progression of the left visual field cut to a complete left homonymous hemianopsia. Magnetic resonance imaging (MRI) of the head showed a right occipital lobe infarct. Results of a cerebral arteriogram were normal. Treatment with aspirin 81 mg once daily was added.
Ten months later the patient was examined by a neurologist because of daily headaches in the bitemporal and vertex regions; intermittent dizziness, especially when bending over; occasional episodes of left facial and intermittent fingers and feet numbness; progressive deterioration of the memory; and worsening of the vision bilaterally. Neurologic examination demonstrated bilateral homonymous hemianopsia, slightly wide-based and hesitant gait with moderate difficulty on tandem walking and some difficulty balancing on 1 foot, subjective decrease in pin-prick sensation, and some decrease in vibratory sensation in the feet and toes. ESR was 55 mm/hour. Serum antinuclear antibodies and anticardiolipin antibodies were absent. There was no electrophysiologic evidence of a peripheral neuropathy.
Duplex carotid ultrasonography was unremarkable. Contrast-enhanced MRI of the head showed bilateral occipital lobe infarcts and scattered small infarcts within both cerebellar hemispheres. There was also a lacunar infarct within the right lateral basal ganglia with extension of the infarct into the deep white matter of the right frontal lobe. Intracranial magnetic resonance angiography raised the question of high-grade stenosis within the high cervical left vertebral artery proximal to the origin of the posterior inferior cerebellar artery (PICA). There was also decreased flow within the left posterior cerebral artery, whereas the right posterior cerebral artery was not identified (occlusion or very slow flow). Conventional cerebral arteriography demonstrated a marked concentric narrowing of the P1 and P2 segments of the right posterior cerebral artery with no visualization of posterior cerebral artery branches distally. Similar marked concentric narrowing was noted within the right vertebral artery just proximal to the right PICA origin. Areas of concentric narrowing were also noted within the left pericallosal artery in the region of the genu and anterior body of the corpus callosum. Two other band-like strictures involving the left vertebral artery were noted ∼2 cm proximal to the left PICA origin. These findings were all suggestive of CNS vasculitis. Furthermore, some beading of the proximal right superficial temporal artery and right internal maxillary artery was suggestive of changes of GCA. The carotid bifurcations bilaterally were normal.
A second temporal artery biopsy specimen (2 cm) was obtained from the patient, which showed active GCA. A diagnosis of intracranial involvement in active GCA was made and prednisone was increased from 5 mg/day to 25 mg/day. The patient's symptoms promptly resolved and she was asymptomatic at 4-month clinical followup.
Case 2.
A 72-year-old woman was hospitalized because of a 3-month history of progressive impairment of mental status, increasing confusion with progression to stupor, obtundation, gait ataxia, dysarthria, new headache, and jaw claudication. Speech examination revealed muteness reflecting a combination of spastic dysarthria and apraxia of speech on the phonatory level.
Laboratory test results were as follows: ESR was 120 mm/hour, and blood coagulation studies and serum anticardiolipin antibodies were negative. A lumbar puncture was performed, which showed a white cell count of 17 cells/ml with 93% lymphocytes, 5% monocytes, and 2% neutrophils. Cerebrospinal fluid (CSF) protein was elevated at 73 mg/dl, while CSF glucose level was 73 mg/dl. CSF VDRL test results, fluorescent treponemal antibody absorption test results, and results of cultures and smear for acid-fast bacilli were all normal. Transesophageal echocardiogram was negative. Left temporal artery biopsy sample (2.5 cm) showed the presence of GCA, with a typical inflammatory infiltrate with presence of scattered giant cells (pictures of biopsy sample not included). Contrast-enhanced MRI of the head demonstrated the presence of multiple areas of abnormal T2 signal in both cerebellar hemispheres, pons, and the mid-brain consisting of evolving infarctions.
Conventional cerebral angiography demonstrated a high-grade stenosis, 2 cm in length, involving the most superior aspect of the right vertebral artery proximal to the basilar artery. Less advanced segmental narrowings were noted at the origin of the right thalamostriate branches and in the proximal superior cerebellar artery. Carotid arteries were normal in appearance.
Therapy with prednisone 60 mg/day and cyclophosphamide 100 mg/day was started. The patient improved and became more alert; her general strength also improved but she continued to be dependent on others for all of her care. One week after beginning therapy, her ESR was 13 mm/hour. However, 10 days later she suddenly became very lethargic and unresponsive and entered a comatose state. Head computed tomography at high resolution showed a large focus of decreased attenuation within the right cerebral hemisphere as well as diffuse foci of decreased attenuation in the middle cerebellar peduncles, most likely due to ischemia. Methylprednisolone pulse therapy 500 mg intravenous daily for 3 days was started and the dosage of cyclophosphamide was increased to 150 mg/day. However, the patient's condition deteriorated rapidly, and she died 5 days later. Postmortem examination revealed active intracranial vasculitis located in the distal right vertebral artery 1.0 cm proximal to basilar origin (Figure 1). There was no evidence of vasculitis in the carotid arteries or in the extracranial portions of the vertebral arteries. Multiple acute and subacute extensive ischemic infarcts involving the diencephalon, mesencephalon, and metencephalon including the left subthalamic nucleus, left lateral thalamus, left red nucleus and subthalamus, right and left cerebral peduncles, right and left lateral basis pontes, right and left middle cerebellar peduncles, and right and left inferior cerebellar hemispheres were also observed.
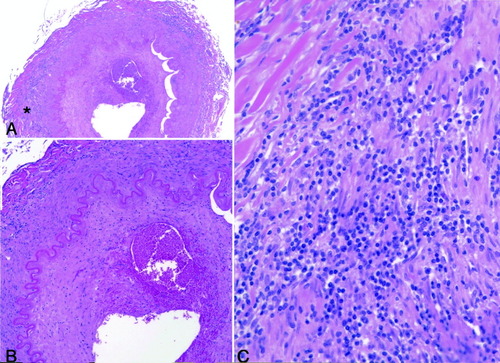
The findings of the intradural portion of the distal right vertebral artery, 1.0 cm proximal to basilar origin (hematoxylin and eosin stain). In A and B, the arterial lumen is markedly narrowed, the intima is thickened and shows recent focal hemorrhage (original magnification × 80) (B). The internal elastic lamina is still intact. Even in low power (original magnification × 40) (A), the media shows multifocal chronic inflammation composed, as best seen in high power (original magnification × 400) (C), by a predominantly lymphocytic infiltrate. No giant cells, as seen in the original superficial temporal artery biopsy (not illustrated), were present at this level. *The portion of the wall illustrated in high power (× 400) in C.
Discussion
GCA is a disease process that is located mainly in medium and large extracranial vessels. Although involvement of internal carotid and vertebral arteries has been reported (3-9), the intracranial/intradural portions of the major vessels are spared. In 1972, Wilkinson and Russell reviewed medical records of 4 individuals they had treated and then reviewed 8 additional cases in the literature that had histologic evidence of GCA in the superficial temporal arteries and a full postmortem examination (4). They described the topographic distribution of histologic changes of intra- and extracranial vessels. Vertebral arteries were more likely to be affected than internal carotid arteries. The arteritic involvement in the vertebral artery ceased abruptly a few millimeters distal to the point of dural perforation; similarly, the internal carotid artery was never involved after perforation of the dura at the base of the brain. The intracranial arteries were not found to be involved in the vasculitic process.
The intracranial arteries have extremely thin walls with much less elastic fibers in the media and adventitia and absent vasa vasorum. The gross diminution or total loss in the amount of elastic tissue occurs when internal carotid and vertebral arteries become intradural. Wilkinson and Russell correlated the pattern of arteritic involvement with the amount of elastic tissue in the media and adventitia in the artery walls (4, 10). They suggested that the underlying pathologic process in GCA may be an autoimmune reaction involving arterial elastic tissue, which could account for the sparing of the intracranial arteries that have little or no elastic tissue in their 2 outer coats.
A subgroup of patients with GCA has been observed to have cerebral ischemic events during the course of the disease. Cerebrovascular accidents have been reported in 3–4% of patients with GCA (1, 2). However, cerebrovascular accidents commonly occur in elderly patients, including those with GCA. This finding makes etiologic relationships only presumptive, although in some cases the temporal relationship suggests a causal link.
Involvement of extradural internal carotid and/or vertebral arteries is usually responsible for cerebrovascular accidents by reduced perfusion related to severe obstruction and/or occlusion. Embolization from thrombosed vessels damaged by arteritis or direct extension of the thrombus from the site of arteritis into the supply artery may also be additional causes of cerebrovascular accidents in patients with GCA (3-9).
Intracranial vasculitis as the direct cause of cerebrovascular accidents in patients with GCA has been reported occasionally. However, it is difficult to evaluate most of these previous reports because the presence of intracranial vasculitis was not clearly documented by pathologic examination and/or angiography. In addition, many cases did not have histologic evidence of GCA in superficial temporal arteries. Some of the cases appear more typical of PCNSV than of GCA with intracranial vasculitis.
There are a number of similarities between GCA and PCNSV. The most common presenting symptom in both conditions is headache; furthermore, the histopathologic features are similar, showing granulomatous vasculitis with giant cells in the vessel walls. Therefore, particularly in the old literature, there has been nosologic confusion between GCA with intracranial vasculitis and PCNSV (11, 12).
In 1951, Cardell and Hanley reviewed all the fatal cases of GCA (3). Full postmortem reports were available for 12 of the 27 reported cases. Although most of these subjects died from cerebrovascular events, intracranial vasculitis was never present. In 1972, Östberg reviewed the necropsy materials of 14 Scandinavian subjects with a diagnosis of temporal arteritis who underwent an adequate postmortem examination in order to evaluate morphologic changes in the arteries (13). Two of the 7 subjects who had evidence of GCA in the temporal artery specimens died from extensive brain infarction. One of these subjects had evidence of thrombosis of the left internal carotid artery; intracranial vasculitis was not observed in either subject.
We searched the literature for patients with histologic evidence of GCA in the superficial temporal artery that also had intracranial vasculitis findings clearly documented using angiography and/or histologic examination (14-20). The clinical data of the 7 patients satisfying these findings are shown in Table 1. Including our 2 patients, 9 patients are described in the literature, and 7 of them died from conditions related to the cerebrovascular events. The presence of intracranial vasculitis in GCA characterizes a group of patients whose disease tended to follow a catastrophic and fatal course. In 7 cases, corticosteroids failed to stop the progressive course. Only 1 patient was treated with immunosuppressive agents. However, most of the cases were identified at autopsy, therefore introducing a selection bias towards the most severe cases. A more favorable course in some patients with intracranial GCA who are not diagnosed may escape attention.
Similar to the observations of Calabrese and coworkers in PCNSV, the cases confirmed by angiography alone appeared to have had a more benign course (12). In fact, the 2 cases that were defined by angiography (Tables 2 and 3) had a disease responsive to corticosteroids with favorable neurologic outcome, whereas the 7 cases with histologic evidence of cerebral vasculitis had a fatal disease unresponsive to corticosteroids. There was no evidence that our patients had some other form of vasculitis with CNS involvement.
| Reference | Age, sex | Neurologic manifestations | Brain CT/MRI | Angiography | Histologic examination† |
|---|---|---|---|---|---|
| Büttner et al (14) | 65, male | Headache, hemiparesis, coma | Multiple infarctions | NP | + (autopsy) |
| Enzmann and Scott (15) | 51, male | Headache, hemiparesis, lethargy, memory deficit | Frontal infarction | + | NP |
| Mclean et al (16) | 85, female | Headache, ataxia, vomiting | − | − | + (autopsy) |
| Thystrup et al (17) | 72, male | Headache, occipital blindness | Bilateral occipital cortex infarctions | NP | + (autopsy) |
| Säve-Söderbergh et al (18) | 87, female | Dizziness, confusion, coma | NP | NP | + (autopsy) |
| Kjeldsen and Reske-Nielsen (19) | 61, female | Headache, diplopia, confusion, lethargy, aphasia, hemiparesis | NP | + | + (autopsy) |
| Gibb et al (20) | 76, male | Headache, leg weakness, urinary retention, drowsiness, hemianopsia, Horner's syndrome, facial paralysis, decreased consciousness, respiratory arrest | Mild atrophy | − | + (autopsy) |
| Salvarani et al (current case) | 67, female | Headache, hemianopsia, TIAs, dizziness, memory decline | Multiple infarctions | + | NP |
| Salvarani et al (current case) | 72, female | Confusion, stupor, cognitive decline, ataxia, dysarthria, headache | Multiple infarctions | + | + (autopsy) |
- * Only cases with biopsy-proven giant cell arteritis and intracranial vasculitis documented using angiography and/or histologic examination. CT = computed tomography; MRI = magnetic resonance imaging; NP = not performed; TIA = transient ischemic attack.
- † Histologic evaluation of intracranial vasculitis.
| Reference | ESR at diagnosis, mm/hour | Therapy | Response to steroids | Cause of death |
|---|---|---|---|---|
| Büttner et al (14) | 95 | Prednisone 100 mg/day | Death | Cerebrovascular complications and sepsis 3 weeks after start of therapy |
| Enzmann and Scott (15) | 44 | Prednisone 60 mg/day | Partial recovery | – |
| Mclean et al (16) | 32 | No therapy | Death | Cerebrovascular complications 3 weeks after start of clinical manifestations |
| Thystrup et al (17) | NP | Prednisone 75 mg/day | Death | Cerebrovascular complications 9 days after start of therapy |
| Säve-Söderbergh et al (18) | 20 | Prednisolone 60 mg/day | Death | Cerebrovascular complications 3 days after start of therapy |
| Kjeldsen and Reske-Nielsen (19) | 45 | Prednisone 30 mg/day | Death | Cerebrovascular complications a few days after start of therapy |
| Gibb et al (20) | 84 | Prednisolone 80 mg/day | Death | Cerebrovascular complications 3 days after start of therapy |
| Salvarani et al (current case) | 55 | Prednisone 25 mg/day | Complete recovery | – |
| Salvarani et al (current case) | 120 | Prednisone 60 mg/day + cyclophosphamide 100 mg/day | Death | Cerebrovascular complications 3 weeks after start of therapy |
- * Only cases with biopsy-proven giant cell arteritis and intracranial vasculitis documented using angiography and/or histologic examination. ESR = erythrocyte sedimentation rate; NP = not performed.
In the first patient, cerebral vasculitis was diagnosed by angiography. Although the value of angiography is limited by poor specificity (12), our patient had high probability findings characterized by segmental narrowing affecting multiple cerebral arteries in the absence of proximal changes suggestive of atherosclerosis. The clinical context and the absence of other conditions that could have accounted for the aforementioned angiographic features thus highly supported the diagnosis of cerebral vasculitis. The second patient had an abnormal angiogram as well as pathologic evidence of vasculitis. Although vasculitis was limited to the right vertebral artery, the segment involved was a deep intracranial portion, and there was no evidence of vasculitis in the extracranial portions of the internal carotid and vertebral arteries. Therefore, we are confident that this patient also had intracranial vasculitis. GCA may occasionally affect the extradural vertebral and internal carotid arteries (3-9), but the only intracranial area that has been documented to be involved includes the first few millimeters of the vertebral arteries as they enter the dura (4). We excluded a third patient with biopsy-proven GCA because she had evidence of extradural involvement of the internal carotid and vertebral arteries proximally to the dural entry point (5).
In conclusion, obstruction and occlusion of internal carotid and/or vertebral arteries are the most frequent causes of cerebrovascular ischemic events in patients with GCA. Arteritic involvement of intracranial/intradural arteries in patients with GCA is a rare event and appears to represent a subset of GCA with a fatal course that fails to respond to corticosteroids.




