Interleukin-6 and chemokines in the neuropsychiatric manifestations of systemic lupus erythematosus
Abstract
Objective
To define the cytokine and chemokine profile in cerebrospinal fluid (CSF) from patients with neuropsychiatric systemic lupus erythematosus (NPSLE).
Methods
Forty-two SLE patients who had been hospitalized because of NP manifestations were studied. Patients were evaluated at hospitalization and 6 months later; a CSF sample was obtained at each evaluation. As controls, CSF from 6 SLE patients with septic meningitis, 16 SLE patients with no history of NP manifestations (non-NPSLE), and 25 patients with nonautoimmune diseases were also studied. Soluble molecules, including cytokines (interleukin-2 [IL-2], IL-4, IL-6, IL-10, tumor necrosis factor α [TNFα], and interferon-γ [IFNγ]) and chemokines (monocyte chemotactic protein 1 [MCP-1], RANTES, IL-8, monokine induced by IFNγ [MIG], and interferon-γ–inducible 10-kd protein [IP-10]), were measured with the use of cytometric bead array kits.
Results
CSF levels of the following molecules were significantly increased in NPSLE patients as compared with non-NPSLE and nonautoimmune diseases control patients, respectively: IL-6 (32.7 versus 3.0 and 2.96 pg/ml), IL-8 (102.8 versus 29.97 and 19.7 pg/ml), IP-10 (888.2 versus 329.7 [P not significant] and 133.6 pg/ml), RANTES (3.8 versus 2.5 and 2.2 pg/ml), MCP-1 (401.7 versus 257.9 [P not significant] and 136.9 pg/ml), and MIG (35.4 versus 11.4 and 3.5 pg/ml). Low levels of IL-2, IL-4, IL-10, TNFα, and IFNγ were found in all groups. All cytokines and chemokines, except TNFα, were significantly higher among the SLE patients with septic meningitis than among the NPSLE patients. Six months later and in the absence of NP manifestations, all elevated molecule levels, except RANTES, in patients with NPSLE had decreased significantly, and no differences were noted between the NPSLE and non-NPSLE groups.
Conclusion
A central nervous system response composed of IL-6 and chemokines, but not Th1/Th2 cytokines, is associated with NP manifestations in SLE patients.
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease of unknown etiology with heterogeneous clinical manifestations, including diverse neuropsychiatric (NP) manifestations (1). The prevalence of NP manifestations in patients with SLE (NPSLE) ranges between 14% and 75%, depending on the ascertainment method used (2). A wide array of NP manifestations has been described, which encompass common features, such as headache and mood disorders, as well as rarer events, such as seizures and psychosis. However, the attribution of NP manifestations in these patients is complex because SLE and non-SLE factors often coexist (3). Several studies have reported that NP manifestations are the most common cause of irreversible damage in SLE (4-7).
Despite these facts, an accurate indicator of lupus involvement of the central nervous system (CNS) has not yet been identified. A variety of clinical, laboratory, and radiographic findings have been reported to be potentially useful. However, because of the multiple pathogenic mechanisms underlying the NP manifestations in SLE (8), the search for an accurate diagnostic test is of utmost importance.
Some studies have shown that cytokines, such as interleukin-6 (IL-6), and chemokines, such as IL-8, are useful for diagnosing NPSLE (9-13). A few other reports have described increased levels of IL-1, IL-10, tumor necrosis factor α (TNFα), and interferon-γ (INFγ) in the cerebrospinal fluid (CSF) of patients with NPSLE (9, 11, 12). More recently, a role for monocyte chemotactic protein 1 (MCP-1)/CCL2, interferon-γ–inducible 10-kd protein (IP-10)/CXCL10, and fractalkine (CX3CL1) has been implicated in NPSLE, suggesting a chemokine-mediated inflammatory reaction in the CNS of NPSLE patients (14-16). Overall, the direct and indirect effects of several mediators of inflammation have been emphasized as possible contributors in the pathogenesis of NPSLE.
The aim of the present study was to measure the levels of representative inflammatory molecules, including cytokines (IL-2, IL-4, IL-6, IL-10, TNFα, and IFNγ) and chemokines (MCP-1/CCL2, IL-8/CXCL8, IP-10/CXCL10, monokine induced by IFNγ (MIG)/CXCL9, and RANTES/CCL5), in CSF samples from NPSLE patients in order to identify the most reliable molecule(s) associated with CNS involvement.
PATIENTS AND METHODS
Study population.
We studied 42 patients with SLE diagnosed according to the American College of Rheumatology (ACR) criteria (17) who had been hospitalized at our institution between February 1, 2003 and June 30, 2005 because of NP manifestations and from whom a CSF sample had been obtained. All NPSLE patients were evaluated according to a standardized protocol by the participating rheumatologists and neurologists at the time of hospitalization and 6 months later. At the time of hospitalization, information about sociodemographic features, SLE characteristics (i.e., age at diagnosis [defined as the date the fourth lupus criterion was met], disease duration at the index hospitalization, number of SLE criteria accumulated, autoantibody profile, etc.), and treatment was gathered using a standardized format. Disease activity was assessed at baseline and 6 months later using the Systemic Lupus Erythematosus Disease Activity Index 2000 update (SLEDAI-2K) (18). The medical records of all patients were reviewed to collect additional information, including the SLE course and chronic damage accrual according to the Systemic Lupus International Collaborating Clinics/ACR Damage Index (19).
Neuropsychiatric manifestations were classified according to the ACR nomenclature and case definitions for neuropsychiatric lupus syndromes (20). Manifestations were attributed to SLE based on the absence of exclusion factors for the attribution of the NP manifestations (20), and none of the patients had minor NP events that have previously been reported to occur at a comparable frequency in the normal population (21).
A CSF sample was obtained from all patients upon their admission to the hospital. A second CSF sample was obtained from 30 of the 42 NPSLE patients 6 months later. As controls, we also studied CSF samples obtained from 6 SLE patients with septic meningitis, 16 SLE patients without current or past NP manifestations (non-NPSLE group), and 25 patients with nonautoimmune diseases and without NP manifestations (nonautoimmune diseases group) who, during the study period, underwent elective surgery that required spinal block and gave their written permission for collection of a CSF sample.
CSF samples were centrifuged at 12,000g, and the supernatant was immediately (<30 minutes in all instances) collected and frozen at −86°C until assayed for cytokine and chemokine contents.
The study was approved by the Institutional Committee of Biomedical Research. All patients gave their written informed consent.
Flow cytometric detection of cytokines and chemokines.
Soluble molecules were measured with the use of cytometric bead array kits (BD Biosciences, San Diego, CA) according to the manufacturer's recommendations. These soluble molecules included Th1 and Th2 cytokines (IL-2, IL-4, IL-6, IL-10, TNFα, and IFNγ) and chemokines (CCL2/MCP-1, CCL5/RANTES, CXCL8/IL-8, CXCL9/MIG, and CXCL10/IP-10).
Bead flow cytometry allows the simultaneous quantification of various proteins in the same test. These assays use beads of the same size that can be distinguished by different fluorescence intensities. Each cluster of the same fluorescence intensity is coated with an antibody against the target molecule. The reaction is revealed by the corresponding secondary antibody conjugated with a different fluorochrome. Fifty microliters of CSF per test was used. Samples were analyzed in a FACScan flow cytometer (Becton Dickinson, San Jose, CA) using the BD Cytometric Bead Array software (BD Biosciences). Results are expressed as picograms per milliliter.
Statistical analysis.
Categorical variables were compared using chi-square or Fisher's exact test. Continuous variables were analyzed using Student's t-test, Mann-Whitney U test, Wilcoxon's signed rank test, paired t-test, or one-way analysis of variance. Cytokine and chemokine values are presented as the median and interquartile range. P values less than 0.05 (2-tailed) were considered significant. Analysis was performed using the SPSS 12.0 computer program (SPSS, Chicago, IL).
RESULTS
Characteristics of the study population.
The mean ± SD age of the study patients was 30.5 ± 11.5 years in the NPSLE group, 37.8 ± 9.8 years in the non-NPSLE group, 29.0 ± 10.9 years in the SLE with septic meningitis group, and 38.6 ± 17.0 years in the nonautoimmune diseases group. Disease characteristics of the SLE patient groups are shown in Table 1.
| NPSLE patients (n = 42) | Non-NPSLE patients (n = 16) | SLE patients with septic meningitis (n = 6) | P | |
|---|---|---|---|---|
| Age, years | 30.5 ± 11.5 | 37.8 ± 9.8 | 29 ± 10.9 | 0.07 |
| No. male/female | 6/36 | 2/14 | 0/6 | – |
| SLE duration, years | 3.8 ± 4.4 | 8.8 ± 6.5 | 3.8 ± 3.7 | 0.009 |
| No. of SLE criteria met | 6.0 ± 1.9 | 6.3 ± 1.9 | 5.5 ± 1.4 | 0.69 |
| SLEDAI-2K score | 14.1 ± 8.7 | 7.0 ± 5.9 | 11.5 ± 7.0 | 0.013 |
| SLICC/ACR Damage Index | 0.7 ± 1.1 | 1.0 ± 1.2 | 0.3 ± 0.5 | 0.40 |
| % taking prednisone | 82 | 81 | 60 | 0.51 |
| Prednisone dosage, mg/day | 16.7 ± 14.2 | 16.6 ± 18.8 | 16.5 ± 25.0 | 0.99 |
| % taking immunosuppressants | 59 | 38 | 40 | 0.33 |
- * Except where indicated otherwise, values are the mean ± SD. SLE = systemic lupus erythematosus; NPSLE = neuropsychiatric SLE; SLEDAI-2K = Systemic Lupus Erythematosus Disease Activity Index 2000 update; SLICC/ACR = Systemic Lupus International Collaborating Clinics/American College of Rheumatology.
The NP manifestations observed were seizure disorders in 14 patients, severe refractory headache in 8, acute confusional state in 8, cerebrovascular disease in 4, mononeuritis multiplex in 3, psychosis in 2, transverse myelitis in 1, polyneuropathy in 1, and pseudotumor cerebri in 1 patient. SLE was considered to be the likely cause of these NP events; in 22 patients (52%), no associated factors for the NP manifestations were identified, and in 20 patients (48%), concurrent, nonexclusion factors (i.e., severe infections, metabolic abnormalities, thrombotic thrombocytopenic purpura, antiphospholipid syndrome, high doses of steroids, valvular heart disease, and arterial hypertension) were identified (20).
Nonautoimmune diseases patients underwent elective surgery because of the following conditions: bone marrow donation in 7 patients, hysterectomy in 6, Tenckhoff catheter placement in 3, hydrocele in 2, lower limb amputation due to type 2 diabetes mellitus in 2, saphenectomy in 2, circumcision in 1, umbilical hernia in 1, and inguinal hernioplasty in 1 patient.
The microorganisms responsible for septic meningitis in the 6 SLE patients were as follows: Streptococcus pneumoniae in 2 patients, Listeria monocytogenes in 1, Cryptococcus neoformans in 1, Mycobacterium tuberculosis in 1, and Staphylococcus species in 1 patient.
Chemokine and cytokine levels.
NPSLE patients versus patients with nonautoimmune diseases.
Since there are no CSF reference values for the molecules studied, we first compared the NPSLE patient group with the group of patients with nonautoimmune diseases. Of all cytokines measured, only IL-6 showed a significant difference, being higher in the NPSLE patients (P = 0.0001). The levels of IL-2, IL-4, IL-10, TNFα, and IFNγ were either low or undetectable in most patients (Table 2).
| Cytokine, patient group | Median (IQR) pg/ml |
|---|---|
| Interleukin-2 | |
| NPSLE | 0 (0–2.2) |
| Non-NPSLE | 0 (0–1.7) |
| SLE with septic meningitis | 2.7 (1.8–71)† |
| Nonautoimmune diseases | 1.3 (0–2.3) |
| Interleukin-4 | |
| NPSLE | 0 (0–8) |
| Non-NPSLE | 0 (0–2.3) |
| SLE with septic meningitis | 34 (7–251)† |
| Nonautoimmune diseases | 1.4 (0–3.7) |
| Interleukin-6 | |
| NPSLE | 32 (3.8–129) |
| Non-NPSLE | 3 (1.3–5.7)† |
| SLE with septic meningitis | 453 (61–8,468) |
| Nonautoimmune diseases | 2.9 (2–3.9)‡ |
| Interleukin-10 | |
| NPSLE | 1.5 (0–6.3) |
| Non-NPSLE | 1.4 (0–2.1) |
| SLE with septic meningitis | 9.2 (4.6–30.8)§ |
| Nonautoimmune diseases | 1.5 (0–2.2) |
| Interferon-γ | |
| NPSLE | 0 (0–7.7) |
| Non-NPSLE | 0 (0–2.1) |
| SLE with septic meningitis | 12 (3–1,044)§ |
| Nonautoimmune diseases | 1.6 (0–2.5) |
| Tumor necrosis factor α | |
| NPSLE | 0 (0–1.3) |
| Non-NPSLE | 0 (0–0) |
| SLE with septic meningitis | 1.1 (0–56) |
| Nonautoimmune diseases | 0 (0–1.7) |
- * NPSLE = neuropsychiatric systemic lupus erythematosus; IQR = interquartile range.
- † P < 0.01 versus NPSLE patients.
- ‡ P < 0.0001 versus NPSLE patients.
- § P < 0.05 versus NPSLE patients.
In stark contrast with the above, all chemokines we analyzed were significantly higher in the NPSLE group than in the nonautoimmune diseases control group, with IP-10/CXCL10, IL-8/CXCL8, and MIG/CXCL9 showing the greatest differences (P = 0.0001), followed by RANTES/CCL5 (P = 0.0004) and MCP-1/CCL2 (P = 0.0014) (Table 3).
| Chemokine, patient group | Median (IQR) pg/ml |
|---|---|
| IL-8/CXCL8 | |
| NPSLE | 102 (35–272) |
| Non-NPSLE | 29 (21–48)† |
| SLE with septic meningitis | 516 (155–955)‡ |
| Nonautoimmune diseases | 19 (13–24)§ |
| MIG/CXCL9 | |
| NPSLE | 35 (9–513) |
| Non-NPSLE | 11 (5–36)‡ |
| SLE with septic meningitis | 1,073 (61–2,223)‡ |
| Nonautoimmune diseases | 3 (2–6)§ |
| IP-10/CXCL10 | |
| NPSLE | 888 (276–4,407) |
| Non-NPSLE | 329 (190–583) |
| SLE with septic meningitis | 5,107 (4,520–6,236)† |
| Nonautoimmune diseases | 133 (84–164)§ |
| MCP-1/CCL2 | |
| NPSLE | 401 (123–1,263) |
| Non-NPSLE | 257 (165–391) |
| SLE with septic meningitis | 2,803 (401–4,713) |
| Nonautoimmune diseases | 136 (88–177)† |
| RANTES/CCL5 | |
| NPSLE | 3.8 (2.9–8.2) |
| Non-NPSLE | 2.4 (2–3.3)¶ |
| SLE with septic meningitis | 9.4 (5.5–15.8)‡ |
| Nonautoimmune diseases | 2.1 (1.8–4.1)¶ |
- * NPSLE = neuropsychiatric systemic lupus erythematosus; IQR = interquartile range; IL-8 = interleukin-8; MIG = monokine induced by interferon-γ; IP-10 = interferon-γ–inducible 10-kd protein; MCP-1 = monocyte chemotactic protein 1.
- † P < 0.01 versus NPSLE patients.
- ‡ P < 0.05 versus NPSLE patients.
- § P < 0.0001 versus NPSLE patients.
- ¶ P < 0.001 versus NPSLE patients.
NPSLE patients versus non-NPSLE patients.
To establish whether these values were attributable to the SLE or specifically to the neurologic activity of SLE, we compared the NPSLE patients with the non-NPSLE patients. This comparison showed differences in IL-6 (P = 0.0016), IL-8/CXCL8 (P = 0.0016), MIG/CXCL9 (P = 0.04), and RANTES/CCL5 (P = 0.0006) levels. There was also a tendency toward statistical significance for the differences in IP-10/CXCL10 (P = 0.064) and IFNγ (P = 0.057) levels (Tables 2 and 3).
NPSLE patients versus SLE patients with septic meningitis.
Finally, we compared the NPSLE group with the SLE with septic meningitis group as a possible positive control for a clear inflammatory process. Indeed all cytokine and chemokine levels, except TNFα, were higher among the SLE patients with septic meningitis than among the NPSLE patients: IL-2 (P = 0.01), IL-4 (P = 0.002), IL-6 (P = 0.099), IL-10 (P = 0.04), IFNγ (P = 0.018), IP-10/CXCL10 (P = 0.005), MCP-1/CCL2 (P = 0.058), MIG/CXCL9 (P = 0.03), RANTES/CCL5 (P = 0.015), IL-8 (P = 0.0158) (Tables 2 and 3).
Figure 1 shows the data for the most representative chemokines and IL-6 in individual patients in all subgroups.
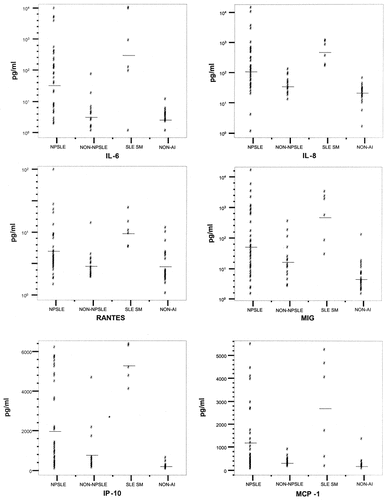
Levels of interleukin-6 (IL-6) and chemokines in cerebrospinal fluid from patients with neuropsychiatric systemic lupus erythematosus (NPSLE), non-NPSLE, SLE with septic meningitis (SM), and nonautoimmune diseases (non-AI). Each data point represents an individual patient. Horizontal lines show the mean. MIG = monokine induced by interferon-γ; IP-10 = interferon-γ–inducible 10-kd protein; MCP-1 = monocyte chemotactic protein 1.
Cytokine and chemokine levels at the time of hospitalization and 6 months later in NPSLE patients.
Six months after hospitalization, a second CSF sample was obtained from 30 of the 42 NPSLE patients. The NP manifestations observed in these 30 patients were seizure disorders in 11, severe refractory headache in 5, acute confusional state in 5, cerebrovascular disease in 2, mononeuritis multiplex in 3, psychosis in 1, transverse myelitis in 1, polyneuropathy in 1, and pseudotumor cerebri in 1. All but 2 patients received prednisone (or its equivalent) at a dosage of 0.5 mg/kg or higher, including 7 patients who received intravenous pulses of methylprednisolone; 5 patients also received intravenous pulses of cyclophosphamide. At the time of the 6-month followup visit, NP manifestations had resolved in all patients (mean ± SD SLEDAI-2K score 5.1 ± 6.4).
As at the time of hospitalization, CSF levels of IL-2, IL-4, IL-10, TNFα, and IFNγ at the 6-month assessment were either low or undetectable, and there was no significant difference between the values obtained at the 2 analyses (data not shown). A clear-cut decrease in nearly all of the previously elevated levels of the study molecules was observed (Table 4). An important result that should be emphasized is that in the second CSF sample, the levels of IL-6 and chemokines in the NPSLE patients were similar to those in the non-NPSLE patients. However, the levels in the NPSLE patients at 6 months were still not as low as those in the nonautoimmune diseases patients at baseline, at least in terms of the values for IL-8 (P < 0.001), MIG (P < 0.0001), IP-10 (P < 0.0001), and MCP-1 (P < 0.0001).
| Cytokine or chemokine | Baseline, median (IQR) pg/ml | 6 months, median (IQR) pg/ml |
|---|---|---|
| IL-6 | 17 (3.8–121) | 3.1 (2.2–4.4)† |
| IL-8/CXCL8 | 106.8 (33.7–272) | 27 (20.5–38.8)‡ |
| MIG/CXCL9 | 48 (9.4–568) | 11.5 (7.1–27)† |
| IP-10/CXCL10 | 888 (313–4,673) | 407 (313–903)§ |
| MCP-1/CCL2 | 401 (184–1,655) | 298 (214–397)¶ |
| RANTES | 4 (3.2–8.2) | 3.8 (3–7.2) |
- * The baseline evaluation was at the time of hospitalization. NPSLE = neuropsychiatric systemic lupus erythematosus; IQR = interquartile range; IL-6 = interleukin-6; MIG = monokine induced by interferon-γ; IP-10 = interferon-γ–inducible 10-kd protein; MCP-1 = monocyte chemotactic protein 1.
- † P = 0.0001 versus baseline.
- ‡ P = 0.001 versus baseline.
- § P = 0.002 versus baseline.
- ¶ P = 0.044 versus baseline.
Figure 2 shows the individual data for the molecules whose levels decreased significantly between baseline and 6 months.
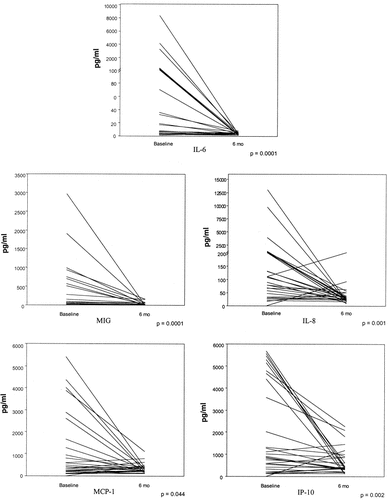
Changes in levels of interleukin-6 (IL-6) and chemokines in cerebrospinal fluid (CSF) from patients with neuropsychiatric systemic lupus erythematosus (NPSLE) between hospitalization (baseline) and 6 months. Paired samples of CSF were obtained from 30 NPSLE patients during active disease and after 6 months, when there was no evidence of NP manifestations. Each line represents an individual patient. MIG = monokine induced by interferon-γ; MCP-1 = monocyte chemotactic protein 1; IP-10 = interferon-γ–inducible 10-kd protein.
DISCUSSION
The mechanisms responsible for the brain damage that occurs during NPSLE have not been clarified. Our results show that despite the diversity and heterogeneous nature of the NP manifestations of SLE, the levels of several molecules are increased in the CSF of most patients during the outbreak of NP activity. Thus, high levels of IL-6 and the chemokines IL-8/CXCL8, MIG/CXCL9, IP-10/CXCL10, MCP-1/CCL2, and RANTES/CCL5 were consistently found in NPSLE patients. The presence of IL-6 and IL-8/CXCL8 in the CSF of patients with NPSLE is one of their commonly described traits (9-13). More recently, MCP-1/CCL2 and IP-10/CXCL10 have been reported in SLE with CNS involvement (14, 15). To our knowledge, MIG/CXCL9 and RANTES/CCL5 have not previously been described in the CSF of patients with NPSLE. With regard to the functions of these molecules, it is worth noting that even though IP-10/CXCL10 and MIG/CXCL9 are predominantly chemoattractive for Th1 cells, MCP-1/CCL2 for Th2 cells, and RANTES/CCL5 for both cell subpopulations (22), we found almost no production of the characteristic cytokines by any of these cells in the NPSLE patients. Th1 and Th2 cytokines were detected only in the patients with SLE with septic meningitis, where there is a clear inflammatory setting and disruption of the blood–brain barrier.
That only IL-6 and chemokines are expressed during the clinical activity of NPSLE clearly reflects a quite different scenario from that found in the peripheral blood, where, besides chemokines, cytokines are conspicuous (23, 24). This difference suggests that the production of these molecules occurs in situ and that the damage seen in NPSLE does not require the involvement of factors derived from blood. In fact, evidence has been obtained suggesting that neurons, microglia, and astroglia can all serve as both targets and sources of chemokines (25, 26), which seems to represent an innate immune response. Moreover, previous reports suggest that enhanced expression of proinflammatory cytokines such as TNFα, IL-1β, and IL-6 is not required for neuronal and glial responses to injury and that chemokines, particularly MCP-1/CCL2, may have another function in the damaged CNS that is distinct from its role in proinflammatory events (27).
These observations, together with our results, which did not show any increase in cytokines (with the exception of IL-6), makes it feasible to speculate that increased chemokine levels, acting either in an autocrine or a paracrine manner, can be involved in the CNS damage in NPSLE, whether by activation and signaling systems or even by other functions of which we are not yet aware. In this regard, recent studies have indicated that in addition to chemotactic activity for leukocytes, MCP-1/CCL2 also plays a role in tumor metastasis and angiogenesis, in the development of CNS, immune, and vascular systems, as well as in the modulation of cell proliferation, apoptosis, and protein synthesis (28-32). Even though MCP-1 has been the best-characterized chemokine so far, it is not unreasonable to suppose that the other chemokines evaluated will also show functions other than inflammatory or cell recruitment, and this might suggest that inflammation is not an obligatory component of the events that initiate or follow NP manifestations in SLE patients.
In a high percentage of NPSLE patients, it was possible to obtain a second CSF sample after the NP manifestations had resolved. Thus, we were able to show that the levels of practically all of the molecules that had originally shown high concentrations had decreased significantly, although not as low as the levels found in the patients with nonautoimmune diseases. Rather, they reached a range of values that could be defined as the basal level in SLE, since they were similar to the levels found in the non-NPSLE patients. This information could prove to be of importance, because SLE patients show neurologic damage over the long-term, which manifests itself as a decrease in brain volume on magnetic resonance imaging (MRI) studies and as a worsening of cognitive function clinically (7, 33). Even though this worsening is greater in patients with a history of NPSLE, it is also found in patients without previous NP manifestations (34). There is no recognized etiology of these alterations; however, it is possible that this slight, but persistent, increase in chemokine levels in the CSF of SLE patients elicits this damage by maintaining a chronic low-level response.
In this regard, previous reports have indicated that in patients with subtle cognitive impairment, there is an increase in IP-10/CXCL10, MCP-1/CCL2, and IL-8/CXCL8 levels (35). Even though current knowledge does not enable us to establish a cause-and-effect relationship between the increase in chemokine levels and the occurrence of neurologic damage, the link between them is amply suggestive that this indeed could be the case. Consistent with this possibility is a recent study showing that the presence of intrathecal IL-6 and IL-8 led to the synthesis of matrix metalloproteinase 9, which potentially culminated in an insult to the brain parenchyma, resulting in the release of neuronal and astrocytic degradation products that terminated in MRI-verifiable lesions and clinical states of brain deficiency (13).
Our results confirm previous hypotheses about the intrathecal presence of cytokines and chemokines in the CSF of patients with NPSLE. However, several issues remain unexplained that deserve consideration. First, what triggers the response observed in NPSLE? Second, why is this response not self-perpetuated like the ones found elsewhere (e.g., the kidney)? Third, why do non-NPSLE patients with relatively high CSF levels of chemokines not show overt clinical manifestations of CNS? Fourth, does the presence of chemokines in the CSF of non-NPSLE patients represent an inflammatory response or an attempt to restore homeostasis against a persistent stimulus? Certainly, many more questions could be posed. However, what seems to be conclusive is that overproduction of IL-6 and chemokines in the CSF plays a key role in the pathogenesis and emergence of NPSLE, although strictly speaking, this is not sufficient to explain it.
Our study has several limitations. Although a relatively large number of patients with NP manifestations were included, we studied the cytokine and chemokine profile for NP manifestations in general but were unable to define the profile for specific NP manifestations, since the study was not adequately powered for such an analysis. Given that we did not have a control group of patients with nonautoimmune diseases and similar NP manifestations, we could not determine whether the abnormal levels of IL-6 and chemokines found were specific for NPSLE patients or were reflective of the NP event itself, regardless of the etiology. We tried to include only patients with NP manifestations that were attributable to SLE, rejecting patients with exclusion factors for the attribution of the NP manifestations (20) as well as patients with minor NP events that have a comparable frequency in the normal population (21). In the latter study, all patients with headaches, both tension-type and migraine, were excluded, but no patient with severe refractory headache was seen. Our study included patients with severe refractory headache, as defined in the SLEDAI-2K, because it is considered a clinical manifestation of lupus activity and because this was the reason for their hospitalization.
In 48% of our patients, there were concurrent non-SLE factors, and their contribution to the development of the hospitalization event is uncertain. Since this is a complex issue, some misclassification in terms of the attribution could be present. Our results apply to patients with NPSLE manifestations who needed to be hospitalized for diagnosis and/or treatment. However, they would not apply to nonhospitalized patients or to chronic serious manifestations (e.g., seizures). Because this study was conducted in a single center with limited ethnic variation among the patients, one must be circumspect about extrapolating the results to all patients with SLE.
Much remains to be learned about the pathogenetic mechanisms that lead to NP manifestations in SLE patients. The absence of experimental models and the difficult access to the CNS are barriers that will have to be cleared through other means. The quantification of IL-6 and chemokines in CSF however, seems to be an accurate indicator of neurologic involvement in SLE that could be useful in the followup and evaluation of treatment response in these patients. It is important to do our utmost to understand and possibly prevent these clinical manifestations of SLE, which undoubtedly, are still one of the major causes of morbidity and irreversible damage.
AUTHOR CONTRIBUTIONS
Dr. Sánchez-Guerrero had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Fragoso-Loyo, Orozco-Narváez, Dávila-Maldonado, Llorente, Sánchez-Guerrero.
Acquisition of data. Fragoso-Loyo, Richaud-Patin, Orozco-Narváez, Dávila-Maldonado, Atisha-Fregoso, Llorente, Sánchez-Guerrero.
Analysis and interpretation of data. Fragoso-Loyo, Richaud-Patin, Orozco-Narváez, Dávila-Maldonado, Atisha-Fregoso, Llorente, Sánchez-Guerrero.
Manuscript preparation. Fragoso-Loyo, Richaud-Patin, Dávila-Maldonado, Atisha-Fregoso, Llorente, Sánchez-Guerrero.
Statistical analysis. Fragoso-Loyo, Atisha-Fregoso, Sánchez-Guerrero.




