Primary malignant lung tumors in children: A report from the Australian Childhood Cancer Registry, 1983-2015
Abstract
Lung cancers in children under the age of 15 are very uncommon, with a scarcity of literature describing patient characteristics and survival. This study assessed first primary malignant cancers occurring in the trachea, bronchus, or lung (International Classification of Diseases for Oncology, 3rd edition [ICD-O-3] codes C33-C34) for the period 1983-2015, using data from the population-based Australian Childhood Cancer Registry. Variables of interest included morphology, sex, age group, and metastatic status at diagnosis. Mode of treatment was also assessed where possible. The Kaplan-Meier method was used to calculate 5-year observed survival. Of the 53 in-scope patients, almost half (n = 23, 43%) were diagnosed with pleuropulmonary blastoma and a further 8 (15%) had a carcinoid tumor. Few of the patients with details available on stage at diagnosis (n = 7 of 43, 16%) presented with metastatic disease. Surgical excision was the most common treatment (30 of 37 children, 81%), with two-thirds (n = 28 of 43, 65%) receiving chemotherapy. Five-year observed survival was estimated to be 74% (95% CI = 61%-85%). Our results represent one of the largest and most complete population-based cohorts of children with primary malignant lung cancers available to date. Detection of childhood lung cancer can be difficult due to the rarity of this disease and symptoms that are typically nonspecific.
Abbreviations
-
- ACCR
-
- Australian Childhood Cancer Registry
-
- CI
-
- confidence interval
-
- ICD-O-3
-
- International Classification of Diseases for Oncology, 3rd edition
-
- IPPBR
-
- International Pleuropulmonary Blastoma Registry
-
- SEER
-
- Surveillance, Epidemiology, and End Results Program
1 INTRODUCTION
Primary malignant lung cancers in children are extremely rare. The International Agency for Research on Cancer estimated that there were around 640 cases diagnosed worldwide in 2018, accounting for only 0.3% of all childhood cancers (ages 0-14) and 0.03% of all lung cancers.1 Consequently, little is known about the epidemiology of childhood primary malignant lung cancer.
Much of the available literature comprises individual case reports, small series of specific tumor subsets, a mix of benign and malignant tumors, and studies including both children and adolescents, making generalization difficult.2 Our aim was to report details of primary malignant lung cancers diagnosed nationally over more than three decades from the population-based Australian Childhood Cancer Registry (ACCR).
2 MATERIALS AND METHODS
We conducted a retrospective cohort study. The ACCR contains details of all children resident in Australia and diagnosed with cancer under 15 years of age,3 with all Australian state and territory population-based cancer registries providing annual information to the ACCR on every child with cancer registered within their jurisdictions. It operates with ethics approval from the University of Queensland Behavioral and Social Sciences Ethical Review Committee (reference number 2004000302) and 15 additional Human Research Ethics Committees representing each of the state/territory cancer registries and major pediatric treating hospitals throughout Australia. A waiver of informed consent has been approved for the ACCR.
Data from the ACCR were available for the period 1983 to 2015 at the time of the study. Treatment details were available for the subset of children who were admitted to a major pediatric hospital and were obtained by examination of hospital medical records.
First primary malignant cancers occurring in the trachea, bronchus, or lung (International Classification of Diseases for Oncology, 3rd edition [ICD-O-3] codes C33-C34) were included, collectively referred to here as “lung cancer.” Second primary tumors were excluded to avoid cases arising as a result of treatment for an earlier cancer.
-
Epithelial neoplasms (including carcinoid tumors and carcinomas);
-
Embryonic tumors (mostly pleuropulmonary blastoma);
-
Mesenchymal tumors (soft tissue sarcomas); and,
-
Lymphohistiocytic cancers (lymphomas and lymphoproliferative disorders).
Frequency counts were produced by morphology, sex, age group, metastatic status at diagnosis, and anatomic site. Differences by type of lung cancer were assessed using Fisher's exact test where feasible. Observed survival was calculated using the Kaplan-Meier method for all patients combined and for the subgroups of epithelial neoplasms and embryonic tumors. For patients who remained alive, survival time was censored at either 5 years after diagnosis or the end of the study period (31 December 2015), whichever occurred first. The log-rank test was used to test for equality of the survival curves.
3 RESULTS
3.1 Patient and tumor characteristics
There were 53 first primary malignant childhood lung cancers diagnosed in Australian children between 1983 and 2015, including 23 embryonic tumors (43%), 18 epithelial neoplasms (34%), 6 lymphohistiocytic cancers (11%) and 6 mesenchymal tumors (11%). In total, these cases represent less than 0.3% of the total of 20 549 childhood cancers registered in Australia between 1983 and 2015. Pleuropulmonary blastoma (ICD-O-3 code 8973/3; n = 23, 43%) and carcinoid tumors (ICD-O-3 code 8240/3; n = 8, 15%) were the two most common individual morphologies. The remaining 22 cases encompassed 16 separate morphologies, underscoring the rarity of many of the tumor subtypes (Table 1).
| ICD-O-3 code | Morphology name |
|---|---|
| Embryonic tumors (n = 23) | |
| 8973/3 | Pleuropulmonary blastoma |
| Epithelial neoplasms (n = 18) | |
| 8010/3 | Carcinoma NOS |
| 8140/3 | Adenocarcinoma NOS |
| 8240/3 | Carcinoid tumor NOS |
| 8246/3 | Neuroendocrine carcinoma NOS |
| 8340/3 | Papilliary carcinoma (follicular variant) |
| 8430/3 | Mucoepidermoid carcinoma |
| 8480/3 | Mucinous adenocarcinoma |
| Lymphohistiocytic cancers (n = 6) | |
| 9680/3 | Lymphoma, large B cell, diffuse NOS |
| 9687/3 | Burkitt lymphoma NOS |
| 9727/3 | Precursor cell lymphoblastic lymphoma NOS |
| 9729/3 | Precursor T-cell lymphoblastic lymphoma |
| 9750/3 | Malignant histiocytosis |
| Mesenchymal tumors (n = 6) | |
| 8830/3 | Malignant fibrous histiocytoma |
| 8910/3 | Embryonal rhabdomyosarcoma NOS |
| 9120/3 | Haemangiosarcoma |
| 9260/3 | Ewing sarcoma |
| 9365/3 | Askin tumor |
- Notes: Pleuropulmonary blastoma includes 10 cases coded as pulmonary blastoma (morphology code 8972/3) before the introduction of morphology code 8973/3.
- Abbreviation: NOS, not otherwise specified.
Girls (n = 29, 55%) slightly outnumbered boys (n = 24, 45%) with no difference in the distribution of sex by morphological category (P = .81). Overall median age at diagnosis was 4 years (interquartile range = 2-11 years), but this varied between those with embryonic tumors (median 2 years, interquartile range = 0-3 years) and patients with epithelial neoplasms (median 12 years, interquartile range = 10-13 years). Of the 45 children in the study cohort for whom metastatic status at diagnosis was recorded, 7 (16%) presented with metastatic disease. No differences were found in the distribution of metastatic disease by tumor subtype (P = 1.00). There were also no clear patterns in anatomic distribution, although noting that the exact site in the bronchus or lung was not specified for more than one-third (n = 20, 38%) of patients.
3.2 Treatment
Among patients for whom treatment data was available, 30 of 43 children (70%) received surgery with curative intent (either complete [n = 25 of 30, 83%] or incomplete [n = 5 of 30, 17%] removal of the tumor); this increased to 81% (30 of 37), however, after excluding those with lymphohistiocytic cancers of the lung, who typically do not undergo surgery (n = 6). Approximately two-thirds of the patients overall (n = 28 of 43, 65%) were treated with chemotherapy; this included the majority (n = 19 of 22, 86%) of patients with embryonic tumors, in contrast to none of 10 children with epithelial neoplasms (P < .001). Adjuvant radiotherapy was recorded for only 5 of the 43 children with treatment details (12%) and was most commonly used for mesenchymal tumors (3 out of 5 patients, 60%).
3.3 Observed survival
A total of 15 children in the study cohort (28%) died by 31 December 2015, including 13 deaths within 3 years of lung cancer diagnosis. Around half (4 of 7, 57%) of those who presented with metastatic disease had died by the end of the study period. Eight of 23 (35%) patients with an embryonic tumor and 5 of 18 (28%) with an epithelial neoplasm died. All of the deaths were attributed to cancer, and all except 1 (n = 14, 93%) were coded to the original lung cancer. Median age at death was 8 years (interquartile range = 5-13 years), with age at death ranging between 4 and 10 years for those with an embryonic tumor compared with 10-22 years for children with an epithelial neoplasm.
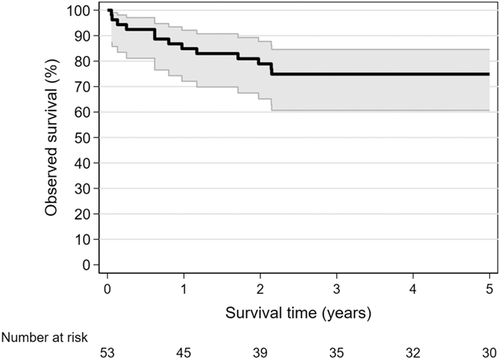
Five-year observed survival following childhood lung cancer diagnosis was estimated at 74% (95% CI = 61%-85%); Figure 1. For the 38 patients who were recorded as being diagnosed with localized disease, 5-year survival was 86% (95% CI = 71%-94%). There was no significant difference (P = .88) in observed survival after 5 years for children with epithelial neoplasms (78%; 95% CI = 51%-91%) compared with those with an embryonic tumor (69%; 95% CI = 45%-84%).
4 DISCUSSION
The data reported in our study represent one of the largest population-based cohorts of children with primary malignant lung cancers in the literature to date. Dishop et. al2 described 25 children (age range: 0-15) with these tumors treated at or referred to the Texas Children's Hospital between 1982 and 2007, including 14 cases (56%) of pleuropulmonary blastoma and three (12%) carcinoid tumors, reasonably similar to the morphological distribution in the ACCR data. In contrast, SEER data for the period 1973 to 2004 contained 52 malignant lung neoplasms in the 0-14 age group, with pleuropulmonary blastoma and carcinoid tumors accounting for 25% and 37% of cases, respectively.4 Possible reasons for the apparent disparity in the mix of tumor types between the SEER and Australian population-based patient cohorts are not clear.
Other studies have focused on specific types of childhood lung cancers. An examination of the National Cancer Data Base in the United States, a nationwide facility-based collection that purports to capture 70% of all newly diagnosed cancers, yielded 211 primary malignant lung tumors of epithelial origin diagnosed in the 0-18 age group between 1998 and 2011.5 About two-thirds (63%) of these patients had carcinoid tumors. Tumor histology was the greatest predictor of survival, with 5-year observed survival varying widely from 26% for adenocarcinoma to 100% for mucoepidermoid carcinoma5; survival was not reported for the entire patient group, however, and so a comparison with our results was not possible.
A review of the International Pleuropulmonary Blastoma Registry (IPPBR),6 a worldwide multicenter collaboration, reported on 350 confirmed cases of pleuropulmonary blastoma between 1962 and 2012. There were no age restrictions on the study, but patients were predominantly under 5 years of age at diagnosis. Cases represented 42 different countries with the majority from the United States. Prognosis was strongly influenced by pathologic type, with 5-year overall survival of 91%, 71%, and 53% for types I (cystic tumors only), II (mix of cystic/solid tumors), and III (solid tumors only), respectively,6 compared with the 69% survival rate reported here for all pleuropulmonary blastoma cases combined.
A strong genetic association for pleuropulmonary blastoma has been reported by other researchers,7, 8 with around two-thirds of tested patients in the IPPBR found to have a germline mutation in the DICER1 gene.6 Loss of function of DICER1 may lead to uncontrolled cell proliferation.6 The cause of most other cases of childhood lung cancer are unknown.
Detection of childhood lung cancers is challenging due to their rarity and variations in presentation.2, 9 Symptoms may include fever, chest or abdominal pain, persistent coughing, hemoptysis, recurrent infections, and bronchial obstruction leading to pneumonitis,9, 10 depending on the type of tumor. These nonspecific symptoms may result in delays in diagnosis and/or misdiagnosis with other respiratory diseases, such as asthma.2
Carcinoid tumors typically have aggressive local growth but metastatic potential is less common.2 In pleuropulmonary blastoma multiple lesions may be present and metastases are more likely with malignant disease.2 As evidenced in these Australian data, treatment for carcinoid tumor of the lung thus centers around complete surgical excision5, 9 whereas the large majority of pleuropulmonary blastoma patients are treated with a combination of surgery and chemotherapy and occasionally radiotherapy.6
A strength of this report is the use of population-based data capturing all relevant cases diagnosed in Australia over the study period. Even so, analysis of trends over time or multivariable modeling were not feasible due to the small number of cases. We were also restricted in the ability to differentiate between subclassifications of pleuropulmonary blastoma or carcinoid tumors as the required clinical information was not collected. Genetic details are also not currently available in the ACCR and so we were unable to perform an assessment of DICER1 mutations within our study cohort.
The information presented here adds to the limited information published on this extremely rare disease. Descriptive and etiological studies in larger populations are required to identify possible risk factors which at present remain largely unknown, especially for epithelial neoplasms.
ACKNOWLEDGMENTS
The authors wish to thank Leisa O'Neill and Chloe Henshaw for their work in the ACCR. We also acknowledge the assistance of all Australian state and territory cancer registries, the Australian Institute of Health and Welfare and each of the major pediatric oncology-treating hospitals throughout Australia.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.




