EAACI Guidelines on Allergen Immunotherapy: Allergic rhinoconjunctivitis
Abstract
Allergic rhinoconjunctivitis (AR) is an allergic disorder of the nose and eyes affecting about a fifth of the general population. Symptoms of AR can be controlled with allergen avoidance measures and pharmacotherapy. However, many patients continue to have ongoing symptoms and an impaired quality of life; pharmacotherapy may also induce some side-effects. Allergen immunotherapy (AIT) represents the only currently available treatment that targets the underlying pathophysiology, and it may have a disease-modifying effect. Either the subcutaneous (SCIT) or sublingual (SLIT) routes may be used. This Guideline has been prepared by the European Academy of Allergy and Clinical Immunology's (EAACI) Taskforce on AIT for AR and is part of the EAACI presidential project “EAACI Guidelines on Allergen Immunotherapy.” It aims to provide evidence-based clinical recommendations and has been informed by a formal systematic review and meta-analysis. Its generation has followed the Appraisal of Guidelines for Research and Evaluation (AGREE II) approach. The process included involvement of the full range of stakeholders. In general, broad evidence for the clinical efficacy of AIT for AR exists but a product-specific evaluation of evidence is recommended. In general, SCIT and SLIT are recommended for both seasonal and perennial AR for its short-term benefit. The strongest evidence for long-term benefit is documented for grass AIT (especially for the grass tablets) where long-term benefit is seen. To achieve long-term efficacy, it is recommended that a minimum of 3 years of therapy is used. Many gaps in the evidence base exist, particularly around long-term benefit and use in children.
Abstract

Abbreviations
-
- AGREE II
-
- Appraisal of Guidelines for Research & Evaluation
-
- AIT
-
- allergen immunotherapy
-
- AR
-
- allergic rhinoconjunctivitis
-
- ARIA
-
- Allergic Rhinitis and its Impact on Asthma
-
- EAACI
-
- European Academy of Allergy and Clinical Immunology
-
- EMA
-
- European Medicines Agency
-
- EPIT
-
- epicutaneous immunotherapy
-
- HDM
-
- house dust mite
-
- ICER
-
- incremental cost-effectiveness ratio
-
- NARES
-
- nonallergic rhinitis with eosinophilia syndrome
-
- QALY
-
- quality-adjusted life years
-
- RCT
-
- randomized controlled trial
-
- SCIT
-
- subcutaneous immunotherapy
-
- SLIT
-
- sublingual immunotherapy
-
- SMD
-
- standardized mean difference
-
- SmPC
-
- summary or product characteristics
-
- SPT
-
- skin prick test
1 INTRODUCTION
Allergic rhinoconjunctivitis (AR) is an allergic disorder of the nose and eyes, resulting in a chronic, mostly eosinophilic, inflammation of the nasal mucosa and conjunctiva.1, 2 Allergic rhinitis, with or without conjunctivitis, is one of the most prevalent allergic diseases affecting around a fifth of the general population.3-5 It is associated with considerable loss of productivity and impaired school performance.6
Allergic rhinoconjunctivitis can usually be diagnosed from its typical presentation (Figure 1). Symptoms include itching, sneezing, watery nasal discharge, and nasal congestion.2 Commonly, there are associated ocular symptoms (watery, red and/or itchy eyes). Symptoms may be described as seasonal and/or perennial; as intermittent or persistent; or mild, moderate or severe according to their impact on the quality of life.8 Symptoms are related to exposure to the offending allergen as well as to nonspecific triggers such as smoke, dust, viral infections, strong odors, and cold air.2 Symptoms on exposure to 1 or more aeroallergens supported by evidence of allergen-specific IgE sensitization to the relevant allergens confirm the diagnosis. AR may co-exist with other forms of rhinitis (Figure 1). Additionally, AR may be associated with symptoms of sinusitis, hearing problems, and asthma.2
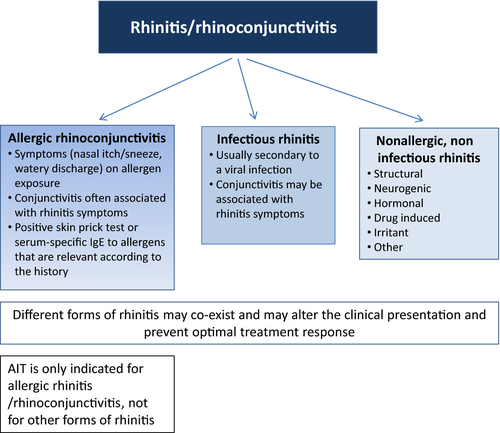
The aims of AR management are to control symptoms and reduce inflammation. Where possible, allergen avoidance can be recommended. Effective allergen avoidance is, however, often not feasible.9, 10 Many patients rely on pharmacotherapy with, for example, oral or topical antihistamines, intranasal corticosteroids, topical cromoglycate, or leukotriene receptor antagonists.2 However, these therapies do not alter the natural history of AR and may also induce side-effects. Additionally, despite medication, a significant number of patients continue to experience symptoms that impair their quality of life. Allergen immunotherapy (AIT) with the subcutaneous (SCIT) or sublingual (SLIT) administration of the culprit allergen(s) may not only desensitize a patient, thereby ameliorating symptoms, but also deliver long-term clinical benefits that may persist for years after discontinuation of treatment.11-13
This Guideline has been prepared by the European Academy of Allergy and Clinical Immunology's (EAACI) Guideline on Allergen Immunotherapy: Allergic Rhinoconjunctivitis Taskforce and is part of the EAACI Guidelines on Allergen Immunotherapy. This Guideline aims to provide evidence-based recommendations for the use of AIT for patients of all ages with allergic rhinitis with or without conjunctivitis. The term AR will henceforth be used to denote either allergic rhinitis or Allergic rhinoconjunctivitis (see Box 1 for definitions of key terms). The primary audience are clinical allergists (specialist and subspecialists); the document may also provide guidance to other healthcare professionals (e.g, physicians from other disciplines, nurses, and pharmacists working across a range of primary, secondary, and tertiary care settings) dealing with AR. The development of the Guideline has been informed by a formal systematic review (SR) and meta-analysis of AIT for AR,14 with systematic review principles being used to identify additional evidence, where necessary.
BOX 1. Key terms
| Allergen immunotherapy (AIT) | Repeated allergen administration at regular intervals to modulate immune response in order to reduce symptoms and the need of medication for clinical allergies and to prevent the development of new allergies and asthma. This is also sometimes known as allergen-specific immunotherapy, desensitization, hyposensitization, or allergy vaccination. |
| Conjunctivitis | Inflammation of the conjunctiva characterized by watery, itchy, red eyes. |
| Efficacy |
Short-term treatment efficacy: clinical benefit to the patient while they are receiving AIT. Long-term treatment efficacy: clinical benefit to the patient for at least 1 y after cessation of the AIT course.14 |
| Rhinitis | Inflammation of the nasal mucosa resulting in at least 2 nasal symptoms: rhinorrhea, blockage, sneezing, or itching. |
| Sensitization | Detectable allergen-specific IgE antibodies, either by means of skin prick test (SPT) and/or specific-IgE antibodies in a serum sample. |
| Subcutaneous immunotherapy (SCIT) | Form of AIT where the allergen is administered as subcutaneous injections. |
| Sublingual immunotherapy (SLIT) | Form of AIT where the allergen is administered under the tongue with formulation as drops or fast-dissolving tablets which are administered through the sublingual route. |
2 METHODOLOGY
This Guideline was produced using the Appraisal of Guidelines for Research & Evaluation (AGREE II) approach,17, 18 a structured approach to guideline production (see Table S1). This is designed to ensure appropriate representation of the full range of stakeholders, a careful search for and critical appraisal of the relevant literature, a systematic approach to the formulation and presentation of recommendations and steps to ensure that the risk of bias is minimized at each step of the process. The process started on April 2015 beginning with detailed face-to-face discussions agreeing on the process and the key clinical areas to address, followed by face-to-face meetings, and regular web conferences in which professional and lay representatives participated.
2.1 Clarifying the scope and purpose of the guidelines
The scope of this EAACI Guideline is multifaceted, providing statements that assist clinicians in the optimal use of AIT in the management of patients with AR and identifying gaps for further research.
2.2 Ensuring appropriate stakeholder involvement
Members of the EAACI Taskforce on AIT for AR represented a range of 18 countries and disciplinary and clinical backgrounds, including allergists (specialist and subspecialists), pediatricians, primary care specialists, ophthalmologists, otolaryngologists, pharmacists, immunologists, nurses, and patient representatives. Methodologists took the lead in undertaking the underpinning SR, while clinical academics took the lead in formulating recommendations for clinical care. Representatives of immunotherapy product manufactures were given the opportunity to review and comment on the draft guidelines as part of the peer review and public comment process at the final stage. These comments were considered by Taskforce members, and, where appropriate, revisions were made.
2.3 Systematic reviews of the evidence
The initial full range of clinical questions that were considered important was rationalized through several rounds of iteration to agree on 1 key question: What are the effectiveness, cost-effectiveness, and safety of AIT in patients with AR? This was then pursued through a formal SR of the evidence by independent methodologists as previously published14, 19; only double-blind RCTs were included in the effectiveness analyses. We continued to track evidence published after our SR cutoff date of October 31, 2015, and, where relevant, studies were considered by the Taskforce chairs. This evidence will formally be considered in the systematic review update that will precede the update of this Guideline (discussed below).
2.4 Formulating recommendations
We graded the strength and consistency of key findings from the SR and performed meta-analyses, using a random-effects model to take into account the heterogeneity of findings.14 These were used to formulate evidence-based recommendations for clinical care20 (Box 2). This involved formulating clear recommendations with the strength of evidence underpinning each recommendation. Where the systematic review did not cover the clinical area, we took a hierarchical approach reviewing other evidence until we could formulate a recommendation, that is: (i) other systematic reviews on the subject to see whether these provided any clarity on the topic; (ii) RCTs within these systematic reviews; (iii) other RCTs known to Taskforce members; and (iv) a consensus-based approach within the Taskforce. This evidence was graded as described in Box 2 using the SR results14 and clearly labeled in the recommendation tables. Recommendations apply to all ages unless otherwise indicated in the tables. When there were insufficient pediatric data, we extrapolated from the adult recommendation where it was biologically likely that the intervention would also be effective in children, but downgraded the recommendation by at least 1 level. Taskforce members identified the resource implications of implementing the recommendations, barriers, and facilitators to the implementation of each recommendation, advised on approaches to implementing the recommendations, and suggested audit criteria that can help with assessing organizational compliance with each recommendation.
BOX 2. Assigning levels of evidence and strength of recommendations
| Level of evidence | |
| Level I | Systematic reviews, meta-analysis, randomized controlled trials |
| Level II | Two groups, non-randomized studies (e.g., cohort, case–control) |
| Level III | One group, non-randomized (e.g., before and after, pretest, and post-test) |
| Level IV | Descriptive studies that include analysis of outcomes (single-subject design, case series) |
| Level V | Case reports and expert opinion that include narrative literature, reviews, and consensus statements |
| Grades of recommendation | |
| Grade A | Consistent level I studies |
| Grade B | Consistent level II or III studies or extrapolations from level I studies |
| Grade C | Level IV studies or extrapolations from level II or III studies |
| Grade D | Level V evidence or troublingly inconsistent or inconclusive studies at any level |
| Strength of recommendations | |
| Strong | Evidence from studies at low risk of bias |
| Moderate | Evidence from studies at moderate risk of bias |
| Weak | Evidence from studies at high risk of bias |
| Recommendations are phrased according to the strength of recommendation: strong: “is recommended”; moderate: “can be recommended”; weak: “may be recommended in specific circumstances”; negative: “cannot be recommended”. | |
| Approach adapted from Oxford Centre for Evidence-based Medicine—Levels of Evidence and Grades of Recommendations.20 The adaptation involved providing an assessment of the risk of bias, based on the Cochrane risk of bias tool, of the underpinning evidence and highlighting other potentially relevant contextual information. | |
2.5 Peer review and public comment
A draft of these guidelines was externally peer-reviewed by invited experts from a range of organizations, countries, and professional backgrounds. Additionally, the draft guideline was made available on public domain on the EAACI Web site for a 3-week period in May 2017 to allow a broader array of stakeholders to comment. All feedback was considered by the Taskforce members and, where appropriate, final revisions were made in light of the feedback received. We will be pleased to continue to receive feedback on this guideline, which should be addressed to the corresponding author.
2.6 Identification of evidence gaps
The process of developing this Guideline has identified a number of evidence gaps which are prioritized (Table 10).
2.7 Editorial independence and managing conflict of interests
This Guideline was funded and supported by EAACI. The funder did not have any influence on the guideline production process, on its contents or on the decision to publish. Taskforce members’ conflicts of interest were declared at the start of the process and taken into account by the taskforce chairs as recommendations were formulated. Final decisions about strength of evidence for recommendations were checked by the methodologists who had no conflict of interests in this area.
2.8 Updating the guidelines
European Academy of Allergy and Clinical Immunology plans to update this guideline in 2022 unless there are important advances before then.
3 GENERAL CONSIDERATIONS BEFORE INITIATING AIT FOR AR
3.1 General considerations
Allergen immunotherapy is only indicated in the presence of symptoms strongly suggestive of AR, with or without conjunctivitis (Table 1).8, 14, 21 Many patients will also have co-existing asthma. There should be symptoms on aeroallergen exposure with evidence of allergen-specific IgE sensitization (positive SPT or serum-specific IgE).14 Identification of the allergen(s) driving symptoms is the first level of patient stratification ensuring that the correct allergen solution is used for AIT. Occasionally, SPT or specific IgE results may not clearly identify the key allergen(s) causing the AR symptoms in polysensitized patients. Component resolved diagnostics may have a role in deciding which aeroallergen(s) should be chosen but definitive trials are awaited. An alternative approach is to use nasal or conjunctival provocation testing to prove the clinical relevance of the allergic sensitization in the relevant (target) organs before initiation of AIT but again definitive evidence is awaited.
| General indications | Key references | Contextual considerations |
|---|---|---|
AIT should be considered when all of these criteria are met:
|
Dhami14 | A diagnosis of AR and evidence of IgE sensitization were entry criteria for RCTs in the systematic review. |
| AIT may also be considered in less severe AR where a patient wishes to take advantage of its long-term effect on AR and potential to prevent asthma with grass pollen AIT |
Kristiansen25 Halken23 |
AIT has the potential to alter the natural history of disease reducing AR symptoms after completing an adequate period of immunotherapy and preventing the development of asthma in the short term, up to 2 y post-AIT. |
| Standardized AIT products with evidence of efficacy in the clinical documentation should be used | Dhami14 |
These products have consistent formulations, and so different batches are likely to have similar effects. The meta-analysis14 reveals a considerable heterogeneity in effectiveness between products, and therefore, a product-specific evaluation of efficacy is recommended. |
- The Summary of Product Characteristics (SmPC) should be checked for licensed indications which may differ between preparations.
Allergen immunotherapy is indicated in those patients with moderate-to-severe symptoms (e.g, Allergic Rhinitis and its Impact on Asthma (ARIA) categories moderate-to-severe intermittent or persistent22), despite avoidance measures and pharmacotherapy, that interfere with their usual daily activities or sleep. AIT may also be considered in cases with less severe AR where the patient wishes to have the benefit of its long-term effect on rhinitis and a potential disease-modifying effect to prevent asthma.23 AIT products with evidence of efficacy for AR should be used when available.11, 24
3.2 Absolute and relative contraindications
Even when AIT is suitable for a patient with AR, clinicians must consider whether there are any specific patient-related absolute or relative contraindications (Table 2), where the risk from AIT may outweigh the expected benefits. The summary of product characteristics (SmPC) should be reviewed for specific contraindications for individual preparations.
| Key references | Contextual considerations | |
|---|---|---|
| The following are considered to be contraindications: | ||
| Uncontrolled or severe asthma | Bernstein31, Bousquet29, Calderon34, Cox28, CSM 198632, Lockey30, Normansell33, Pfaar11; Pitsios27 |
Weak evidence of risk with uncontrolled asthma, active systemic autoimmune disease, and malignancy from case reports or case series of adverse events with AIT. Taskforce considered that these were contraindications to AIT. Though initiation of AIT is contraindicated during pregnancy, an ongoing AIT is permissible when having been well tolerated by the patient in the past |
| Active, systemic autoimmune disorders (unresponsive to treatment) | Cabrera35, Fiorillo37, Pfaar11, Sánchez-Morillas36; Pitsios27 | |
| Active malignant neoplasia | Larenas-Linnemann39, Pfaar11; Wöhrl38 | |
| AIT initiation during pregnancy | Metzger40, Pfaar11 | |
| With the following, AIT should only be used with caution when benefits outweigh potential risks in an individual patient: | ||
| Partially controlled asthma | Virchow41 | One trial with SLIT tablet41 included some subjects with partially controlled asthma without compromising safety; it is important that confirmatory evidence is acquired. |
| Beta-blocker therapy (local or systemic) | Cleaveland44, Hiatt42, Lang45; Pfaar11 | Weak evidence of risk. May compromise a patient's ability to tolerate an episode of anaphylaxis. This must be considered when deciding whether AIT is appropriate. |
| Severe cardiovascular diseases, for example, coronary artery disease | Larenas-Linnemann39; Linneberg46 | |
| Systemic autoimmune disorders in remission or organ specific | Larenas-Linnemann39, Pitsios27 | Weak evidence of risk from case reports, case series of adverse events with AIT or expert opinion based on clinical experience. Taskforce considered that careful consideration on a case-by-case basis with discussion between patient and the treating physician is required before deciding whether or not to commence AIT. |
| Severe psychiatric disorders | Pitsios27 | |
| Poor adherence | Pitsios27; Pfaar11 | |
| Primary and secondary Immunodeficiencies | Larenas-Linnemann39; Pitsios27 | |
| History of serious systemic reactions to AIT | Calderon34; Pfaar 201411 | |
- The Summary of Product Characteristics (SmPC) should also be checked for product-specific contraindications which may differ between preparations.
4 AIT FOR AR: EVIDENCE-BASED, CLINICAL RECOMMENDATIONS
To underpin this guideline, a SR of the AIT literature was undertaken.14 In general, the meta-analysis suggested that both SCIT and SLIT are effective for AR. They were associated with reductions in symptoms and with medication use. There were insufficient data to determine which of SCIT and SLIT are most effective.
Moderate to substantial heterogeneity was observed in some outcomes evaluated in the meta-analysis.14 This heterogeneity can be explained by the study design (particularly the different outcomes used), study population and the products evaluated. There are data to indicate which preparations are most likely to be effective, so an individual product-based evaluation of the evidence for efficacy is strongly recommended before treatment with a specific product is initiated. Not all AIT products provide sufficient data to support their efficacy in clinical practice.14 As a result of this, the recent German, Austrian and Swiss guideline has followed a product-specific approach.11 This approach is more difficult across Europe with differing local regulations47 and availability of products.48 The specific recommendations in this guideline need to be seen in this context; only standardized AIT products with evidence of efficacy in the clinical documentation should be prescribed. The only exception should be orphan allergens where only a few patients are affected; these are discussed below in the specific allergen section.
Subcutaneous immunotherapy is in general recommended for the treatment of AR in children and adults with moderate-to-severe disease that is suboptimally controlled despite pharmacotherapy14 (Table 3). The evidence for short-term benefit for continuous SCIT is stronger for seasonal rhinitis (Grade A for adults) than for perennial rhinitis (Grade B for adults), where fewer studies have been performed and results are more heterogeneous (Table 3). SCIT is recommended for seasonal disease whether pre- or pre/coseasonally (Table 3, Grade A for adults). Pre/coseasonal therapy benefits from a shorter course of treatment but the 1 head-to-head trial suggests that continuous therapy may be more effective.49
| For each recommendation, an individual product-based evaluation of the evidence for efficacy is recommended before treatment with a specific product is initiated given the heterogeneity in the meta-analysis results. The SmPC should also be checked for for product specific recommendations. | |||||||
|---|---|---|---|---|---|---|---|
| Recommendation | Adults | Children and adolescents | Strength of recommendation | Other considerations | Key references | ||
| Evidence level | Grade of recommendation | Evidence level | Grade of recommendation | ||||
| SCIT | |||||||
| Seasonal allergic rhinitis | |||||||
| Continuous SCIT is recommended for seasonal AR for short-term benefit in those with moderate-to severe disease | I | A | I | B | Strong recommendations for adults based on low risk of bias studies.60-62 Moderate recommendation for children as just one open RCT with risk of bias reporting solely pediatric data.63 | Consistent results, low risk of severe systemic allergic side-effects. Most studies reported pediatric and adult data together. |
Dhami14 for example, Adult: Dolz64, Charpin61, Ferrer62, Jutel75, Scadding65, Walker60 Paediatric: Jacobsen63 |
| Pre- and pre-/coseasonal SCIT is recommended for seasonal AR for short-term benefit | I | A | I | B | Strong recommendation for adults based on low risk of bias studies.69-72 Moderate recommendation for children as only combined adult/pediatric data, one study with low risk of bias73 and with one with unclear risk of bias RCTs74 available. | Consistent results in adult studies; low risk of severe systemic allergic side-effects. |
Dhami14 SR, for example, Adult: Balda69, Bodtger70, Bousquet74, Frew58, Varney71, Zenner72 |
| Continuous grass pollen SCIT is recommended for seasonal AR for short- and long-term benefit | I | A | I | B | Strong recommendation for adults based on above evidence plus 2 low risk of bias long-term studies.83, 84 Moderate recommendation for children as one long-term open RCT with risk of bias.63 | A few adult studies and one pediatric study (designed to assess whether SCIT prevents asthma) demonstrating long-term effectiveness. |
Dhami14 SR, for example, Adult: Durham83, James84 Pediatric: Jacobsen63 |
| Perennial allergic rhinitis | |||||||
| Continuous SCIT is recommended for perennial AR due to HDM for short-term benefit | I | B | I | C* | Strong recommendation for adults based on one study with low risk of bias67 plus one with high risk of bias.68 No exclusive pediatric data. Moderate recommendation for children, based on extrapolation from adult studies. | Few small adult studies, considerable heterogeneity66 and risk of systemic allergic side-effects. *Recommendation for children downgraded from B to C due to lack of exclusive pediatric data. | Dhami14 SR, for example, Adult: Dokic67, Ewan68, Varney66 |
| All | |||||||
| Modified (allergoids) and unmodified allergen extracts for pollens and HDM SCIT are recommended for AR for short-term benefit | I | A | I | B | Strong recommendation for adults based on high-quality studies for both modified61, 67, 76-78 and nonmodified60, 61, 69-73, 76, 79, 80 allergen extracts. Weak recommendation for children as no exclusive pediatric randomized, placebo-controlled data. | Consistent results, low risk of severe systemic allergic side-effects. No exclusive pediatric randomized, placebo-controlled data. |
Dhami14 SR, for example, Modified: Ceuppens81, Corrigan77, Dokic67, Klimek78, Riechelmann82 Nonmodified: Balda69, Bodtger70, Brunet76, Charpin61, Frew58, Ortolani79, Scadding65, Varney71, Walker60, Weyer73, Zenner72 Modified and nonmodified: Bousquet74 |
| SLIT | |||||||
| Seasonal allergic rhinitis | |||||||
| Pre-/coseasonal SLIT is recommended for seasonal ARs for short-term benefit | I | A | I | A | Strong recommendation based on high-quality adult86-89 and pediatric90-92, 155, 156 studies. | Consistent results, low risk of severe systemic allergic side-effects. |
Dhami 201714 SR, for example, Adult: Dahl85, Dahl86, Didier56, Durham87, Palma-Carlos96, Worm89 Pediatric: Blaiss99, Bufe98, Caffarelli90, Halken97, Pajno91, Wahn156 |
| Continuous SLIT can be recommended for seasonal AR for short-term benefit | I | A | I | A | Moderate-to-strong recommendation based on low100 and high101, 102 risk of bias adult studies plus low,111 moderate103 and unclear57 risk of bias pediatric studies. | Some heterogeneity between studies particularly pediatric ones, low risk of severe systemic allergic side-effects. |
Dhami14 SR, for example, Adult: Amar100, Ariano101, Creticos93, Panzner102 |
| SLIT with aqueous solutions can be recommended for seasonal AR for short-term benefit. | I | B | I | A | Moderate recommendation for adults based on a mixture of low104 and high101, 105, 106 risk of bias studies. Strong recommendation for pediatrics based on low risk of bias studies.91, 92 | Some heterogeneity between adult studies, low risk of severe systemic allergic side-effects. |
Dhami14 SR, for example, Adult: Ariano101, Bowen105, Feliziani104 |
| SLIT with grass pollen tablets is recommended for AR for short-term benefit. | I | A | I | A | Strong recommendation based on low risk of bias adult86, 87, 108, 109 and pediatric97-99, 111 studies. | Nonimportant heterogeneity between studies, low risk of severe systemic allergic side-effects. |
Dhami14, SR, e.g |
| Grass pollen SLIT tablets or solution with continuous therapy is recommended for AR for long-term benefit. | I | A | I | A | Strong recommendation for adults based on low risk of bias studies.108, 109 One low risk of bias pediatric study110, 111 | Effective up to 2 y after cessation in adults.108, 109 One pediatric study was designed to look at prevention of asthma. |
Dhami14 SR, e.g Pediatric: Valovirta 2011110 and 2017111 Adult and pediatric: Ott145 |
| Perennial allergic rhinitis | |||||||
| SLIT with aqueous solutions may not be recommended for perennial AR for short-term benefit. | I | C* | I | A | *Weak recommendation against use for adults based on just one high risk of bias RCT so only a grade C recommendation.107 Cannot be recommended in children based on 4 negative RCTs and 1 positive RCT. | Low risk of severe systemic allergic side-effects. Studies of low106, 139, 140, 146 and high144 risk of bias suggest that it is not effective in children. |
Dhami14 SR, for example, Adult: Guez107 Pediatric: Bahçeciler139, de Bot146, Hirsch140, Marcucci144, Tari106 |
| SLIT with HDM tablets is recommended for AR for short-term benefit. | I | A | I | A | Strong recommendation based on low risk of bias adult50-54 and mixed adult/pediatric51, 55 studies. | Nonimportant heterogeneity between studies, low risk of severe systemic allergic side-effects. |
Dhami14 SR, e.g Adult: Bergmann53, Demoly52, Mosbech54, Passalacqua50, Passalacqua147 |
| HDM SLIT tablet with continuous therapy can be recommended for AR for long-term benefit. | I | B | - | C* | Moderate recommendation based on one large, low risk of bias study.53 No pediatric data. | One study demonstrates effectiveness for a year post-treatment53; data require replication especially as 3 y therapy required for grass pollen. *No pediatric data, extrapolated from adult data. | Adult: Bergmann53 |
- Continuous: year-round therapy. Preseasonal: before a pollen season. Coseasonal: during a pollen season. Not all AIT preparations are licensed for children and adolescents. Long-term is defined as at least 1 y after cessation of the AIT course. See allergen factors section for other specific allergens.
Subcutaneous immunotherapy may be administered in aqueous formulation (rarely in Europe) or as a depot adsorbed on aluminum hydroxide or tyrosine. SCIT using either unmodified or modified allergen extracts is recommended for treatment of AR and provides short-term benefit (Table 3, Grade A for adults). This is based on evidence from the meta-analysis14 that showed both unmodified allergen extracts (SMD [95% CI] −0.65 [−0.93, −0.36]) and allergoids/polymerized extracts (−0.60 [−0.89, −0.31]) to be effective in reducing symptoms compared to placebo, with additional support from reduced medication requirements and combined symptom-medication scores. Although clinical trials of modified allergens involved shorter courses of treatment, there have been no head-to-head comparisons with unmodified preparations evaluating efficacy or adverse events using a placebo-controlled, randomized design.
In general, SLIT can be recommended for the treatment of seasonal AR in adults and children. SLIT has been shown to provide short-term benefit during therapy with moderate-to-severe disease that is suboptimally controlled despite pharmacotherapy (Table 3).14 SLIT is recommended to be taken either continuously or pre-/coseasonally commencing a minimum of 2 months and ideally 4 months prior to the start of the pollen season (Grade A for adults).
Sublingual immunotherapy may be taken daily either as fast tablets or drops that are retained under the tongue for at least 1 minute and then swallowed (the summary of the SmPC should also be checked for product specific recommendations). Both are recommended (grades A and B, respectively, for adults) based on short-term reductions in symptoms and rescue medication for sublingual tablets for seasonal AR (Table 3). There are only convincing evidence for effectiveness of SLIT tablets in perennial AR (Grade A) (Table 3).
Sublingual grass pollen tablet immunotherapy for at least 3 years is recommended (Grade A) for the short-term treatment of grass pollen-driven AR in adults.86, 87, 108, 109 Sublingual house dust mite (HDM) tablet immunotherapy for at least 1 year is recommended (Grade A) for the short-term treatment of perennial HDM AR in adults.50-55
While higher doses and/or increased cumulative doses may be more effective, they may be associated with more side-effects56-58; decisions on dose in AIT must be made balancing efficacy and side-effects.59
4.1 Other approaches of AIT for AR
Other approaches aim to improve patient convenience and adherence with shorter courses, while improving or maintaining efficacy and reducing the risk of systemic side-effects (Table 4). As such, adjuvants to AIT extracts are possible candidates.112 For example, TLR-4 agonists (Th1-inducing adjuvant monophosphoryl lipid A) in combination with a grass allergoid have demonstrated effectiveness,113 although in a phase 3 trial, efficacy was modest114 (Grade A for adults, B for children) and there are no head-to-head comparisons with conventional preparations. There is also 1 trial demonstrating efficacy for this approach with ragweed pollen172 and one with tree pollen.95 The TLR-9 agonist (Bacterial DNA oligonucleotides containing a CpG motif) fused to Amb a 1, the major allergen of ragweed showed efficacy in a phase 2 trial115 although this was not observed in a subsequent phase 3 trial. The combination of anti-IgE injections with conventional and rush AIT with nonmodified extracts has been proven to be effective with a marked reduction in systemic side-effects in studies of children116 and adults117 (Grade A recommendation). This is an expensive approach, and there is concern as to when and how to discontinue the anti-IgE when AIT maintenance therapy is achieved.118
| For each recommendation, an individual product-based evaluation of the evidence for efficacy is recommended before treatment with a specific product is initiated given the heterogeneity in the meta-analysis results. Where available, the SmPC should also be checked for for product specific recommendations. | |||||||
|---|---|---|---|---|---|---|---|
| Recommendation | Adults | Children and adolescents | Strength of recommendation | Other considerations | Key references | ||
| Evidence level | Grade of recommendation | Evidence level | Grade of recommendation | ||||
|
A combination of the TLR-4 agonist monophosphoryl lipid A with pollen allergoid is recommended for AR |
I | A | III | B | Strong recommendation for adults based on 3 low risk of bias studies.113, 114, 172 Weak recommendation for children.130 | Consistent randomized controlled data; only one ragweed pollen study, others are grass and tree pollen. Only one uncontrolled before and after study pediatric study.130 |
Adult: Drachenberg113, DuBuske114, Drachenberg95, Patel172 Pediatric: Drachenberg130 |
| Combining anti-IgE injections with AIT for AR is recommended for reducing side-effects | I | A | I | A | Strong recommendation based on one low risk of bias adult117 and one low risk of bias pediatric116 study. | Consistent evidence but the required length of cotherapy unclear. |
Adult: Casale117 Pediatric: Rolinck-Werninghaus116 |
| Recombinant AIT can be recommended for birch and grass pollen allergy | I | A | - | B | Moderate recommendation based on 2 double-blind placebo-controlled RCTs of unclear risk of bias.75, 119 | Some evidence of benefit for adults, no pediatric data. | Adult: Jutel75, Pauli119 |
Recombinant AIT is attractive as it allows accurate standardization of allergen products, has potential for personalized therapy based on individual allergen sensitivities and a hypothetical lower risk of inducing new sensitizations. Subcutaneous recombinant birch (Bet v 1)119 and a five-recombinant grass allergen mix75 have been shown to be efficacious with no safety concerns (Grade A for adults, B for children). However, there are no commercially products available at present. A recombinant B-cell epitope-based vaccine, comprising a recombinant hybrid grass allergen mix combined with a hepatitis B domain surface Pre-S protein as an immunologic carrier has shown efficacy in a phase 2 trial.120 T-cell peptide immunotherapy for cat allergy using mixtures of short T-cell epitopes via the intradermal route had promising results in environmental chamber phase 2 studies121; however, it has been reported that a subsequent phase 3 study did not demonstrate effectiveness.122 Studies with other allergen peptide approaches are in progress.124
There has been recent interest in the use of alternative modalities of delivery including the epicutaneous, intradermal and intralymphatic routes. In RCTs, epicutaneous grass pollen immunotherapy (EPIT) has shown modest benefit125 although accompanied by local eczematous reactions at the patch application site. Intradermal grass pollen immunotherapy inhibited allergen-induced cutaneous late responses although in a subsequent RCT, it was ineffective and there was evidence of exacerbation of seasonal outcomes and Th2 inflammation in the skin.126 The intralymphatic route, using a grass pollen extract and a modified cat allergen extract, showed efficacy in some trials127, 128 but not in others.129
5 ALLERGEN FACTORS THAT MAY AFFECT THE EFFICACY OF AIT for AR
5.1 Standardization of allergen extracts
For the common allergens, many companies now provide characterized, standardized, stable preparation for AIT as recommended by EMA.47, 132 For others, such as molds, there are problems with the complexity, variability, and stability of the allergens.133 The lack of standardized extracts may hamper the diagnosis of eligible patients for AIT and may impede the effectiveness of AIT.133, 134 Additionally, nonstandardized preparations may vary between batches increasing the potential for side-effects. Further purification and characterization of such allergens134-136 may result in better extracts for the future. Where possible, standardized allergen products should be used for AIT. Further discussion is available in a position paper on regulatory aspects of AIT.47
5.2 Formulation of SLIT preparations
In deciding on the appropriate preparation to use for AIT, the formulation should be taken into account. For example, 3 large studies have shown efficacy for HDM SLIT tablets,52-54 whereas 3 HDM SLIT studies with sublingual drops were negative,107, 140, 146 and another only demonstrated efficacy in the first and not the second year.50 However, many factors such as differences in allergen content,141 administered volume, number of participants, and statistical power of the study may explain the differences between tablets and drop trials. We recommend that AIT products with evidence of efficacy in the clinical documentation should be used when they are available.
5.3 Allergen mixtures
Both mixtures of grass pollen and mixtures of tree pollen are frequently used in AIT and such an approach is effective.14 The use of different, nontaxonomically related allergens mixed in 1 AIT product has been evaluated in a very limited number of studies. One SCIT study showed that a depigmented-polymerized mixed grass/birch pollen extract was effective over placebo.142 A small study in children demonstrated efficacy using a mixture of grass pollen and HDM SLIT.143 SLIT drops that employed a monomeric Phleum pratense grass pollen extract was more effective when given alone compared to when given in an equivalent dose as part of a combination with a 9-pollen, multi-allergen, sublingual extract.100
There are a number of potential drawbacks of mixing allergens including a dilutional effect, potential allergen degradation due to enzymatic activity of some allergens and the difficulties of adequately demonstrating efficacy of a high number of allergen combinations and/or different products. The EMA has recommended that only homologous allergens (usually ones that are taxonomically related132, for example, a mixture of grass pollen extracts56) should be mixed and that allergens with enzymatic activity (e.g, HDM) should be never used in a mixture. We therefore recommend only homologous allergens to be mixed in AIT preparations until further evidence is available substantiating the efficacy of other mixtures (Grade A) (Table 5) (Table S1). Alternatively, extracts should be given separately.
5.4 Specific allergens
In the recent meta-analysis, there were sufficient SCIT and SLIT studies for subgroup analyses by specific allergens.14 Short-term effectiveness was demonstrated for HDM (symptoms score SMD −0.73; 95% CI −1.37, −0.10), grass pollen (−0.45; −0.54, −0.36), tree pollen (−0.57; −0.92, −0.21), and weed pollen (−0.68; −1.06, −0.30). However, there was substantial heterogeneity for all allergens, particularly molds (−0.56; −2.29, 1.18), suggesting that different preparations may be more or less effective. Before a product is used, an individual product-based evaluation of the evidence for efficacy is recommended.
There are some orphan allergens where robust data from RCTs are sparse or nonexistent. Where there is a clinical need, the available evidence of efficacy and safety needs to be weighed against the needs of the individual patient. Where therapy is considered in the patient's best interest, an early evaluation of its impact on the patient's clinical symptoms is required to determine whether or not therapy should be continued. The generation of controlled clinical trial data to assess efficacy and safety of these orphan products should be encouraged. There will always be orphan allergens where such studies are uneconomic and have to be regulated as named patient products.47
| Recommendation | Adults | Children and Adolescents | Strength of recommendation | Other considerations | Key references | ||
|---|---|---|---|---|---|---|---|
| Evidence level | Grade of recommendation | Evidence level | Grade of recommendation | ||||
| Either a single allergen species or a mixture of well-documented homologous allergens from the same biological family are recommended for patients with AR who are allergic to grass pollens, tree pollens, or HDM | I | A | I | A | Strong recommendations on basis of low risk of bias grass pollen (single grass, e.g,85, 98, 99); mixture of grasses, e.g,56, 145), tree pollen (single tree, e.g,61, 70; mixture of trees, e.g,69) and house dust mite (single, e.g,66; mixture, e.g,147) studies. | Strong RCT evidence that these are effective approaches. Supported by regulators. |
Adult: Balda69, Bodtger70, Charpin61, Dahl85, Didier56, Ott145, Passalacqua147, Varney66, Varney71 Pediatric: Bufe98 |
| Mixtures of allergens from nonrelated biological families are not recommended for AIT. | I | B | — | C* | Strong recommendation against use of allergen mixtures is based on the little available evidence. | No evidence of effectiveness for almost all mixtures. Exception is one positive low risk of bias study in adults (grass and tree pollen mix),142 and this product would therefore be indicated for use for AIT. *No pediatric data, extrapolated from adult data. | |
- Examples of homologous, taxonomically related allergens from the same biological family are the grasses or tree pollens. Also see Table 3.
6 PATIENT FACTORS THAT MAY IMPACT ON THE EFFICACY OF AIT FOR AR
The approach to immunotherapy is a good example of patient stratification. Immunotherapy will only work when directed to the specific allergen(s) driving symptoms. So identifying the driving allergen(s) with a thorough history and assessment of allergic sensitization is an essential example of patient stratification. Not all patients benefit from AIT14 and further stratification approaches to identify the responders would be useful.
6.1 Polysensitized patients
Epidemiological data indicate that most patients with AR are polysensitized.148 Consequently, consideration needs to be given as to whether patients are (i) clinically monoallergic (where only 1 allergen is driving symptoms) and polysensitized or (ii) poly-allergic (symptoms with overlapping exposure to multiple different allergens) and polysensitized. Immunotherapy with a single allergen extract is effective in the first,149 while immunotherapy has been shown to be ineffective150 or less effective in the last situation.151 This may be apparent from the history or may need investigation with component-resolved diagnostics or assessment with nasal or conjunctival provocation challenges where the clinician is experienced in these diagnostic procedures.137 Polysensitized patients who are monoallergic are recommended to receive AIT for the specific allergen that is driving their AR symptoms (Grade A).
For a polysensitized patient who is poly-allergic for homologous (biologically related) allergens (e.g, 2 grass pollens), a single allergen preparation or a mixture of 2 homologous allergens is recommended (Grade B).137 For poly-allergic patients where allergens are not homologous, separate AIT preparations for 1 or 2 of the clinically most important allergens might be recommended with doses given 30-60 minutes apart at separate locations when 2 are selected (Grade C).32, 137 This represents a trade-off between efficacy and safety as both seem to be dose-dependent. More studies are needed to further address this important clinical challenge.
6.2 Co-existing asthma
Co-existing asthma is seen in many participants in the published AR AIT studies.14 Co-existing asthma has no impact on the efficacy of AIT for AR103 and may also lead to improvement in asthma.43 When controlled, mild-to-moderate asthma does not seem to be a safety issue with AIT (Grade A recommendation).41, 43 In 1 large recent asthma SLIT trial, participants with not well-controlled asthma based on an Asthma Control Questionnaire (ACQ-6) were included safely in the study.41 We await confirmatory evidence and emphasize that efforts should be taken to control asthma before commencing AIT. Uncontrolled or severe asthma are definitely considered to be an absolute contraindication to AIT.25-31
6.3 Specific pediatric issues
Similar to adults, AIT should be considered in pediatric patients with AR with evidence of IgE sensitization to clinically relevant allergens (Grade A) (Tables 1 and 3).
The evidence for the efficacy of AIT for AR is limited in children younger than 5 years of age. Some clinical studies have shown the efficacy and safety of both SCIT and SLIT in preschool children,88, 152-155 and children were included from 5 years onward in several of the well-powered SLIT tablet trials.98, 156 Experience suggests that repeated injections of SCIT may be stressful in preschool children. It is recommended that the decision to start the treatment has to be taken on a case-by-case basis together with the patients and their family (Grade D). The decision should depend on several factors, such as the severity of the allergic disease, the clear exposure-symptoms pattern supported by allergic sensitization testing, the impairment of the health-related quality of life and the expected acceptance and adherence to the AIT.
There are more data to drive recommendations for school age children and adolescents although major gaps still exist (Table 3). Many of the SCIT trials are now relatively old, many enrolled only a few children and/or did not present pediatric only analyses. Continuous and pre- and pre/coseasonal SCIT can be recommended (Grade B) for children with seasonal AR (Table 3). Continuous SCIT is also recommended for perennial AR but with a weaker grade due to the lack of exclusive pediatric data (Grade C) (Table 3). There are no exclusive pediatric, placebo-controlled data for allergoid preparations, but 1 controlled trial with a preseasonal treatment regimen has indicated long-term efficacy of preseasonal grass pollen immunotherapy in this age group.157 Two further open RCTs also suggest that SCIT for grass pollen-driven AR does have a long-term benefit.63, 158
For SLIT, there are more recent pediatric trial data to support this approach. In general, pre-/coseasonal and continuous SLIT is recommended for seasonal AR (Grade A) (Table 3). Both tablet and aqueous formulations are recommended (Grade A) (Table 3). There is now one recently published trial supporting the long-term effectiveness for a grass pollen tablet and reduction in asthma symptoms110, 111 (Grade A). For perennial allergic rhinitis, the evidence is not as good. There are no consistent data to recommend SLIT with aqueous solutions for perennial allergic rhinitis, but the SLIT tablet approach has been demonstrated to be effective in the short term in mixed adult/adolescent studies51, 55(grade A).
6.4 Elderly
A detailed allergy history is especially important when evaluating older adults suffering with rhinitis as other types of rhinitis may mimic AR symptoms. There are very few studies specifically evaluating the use of AIT in the elderly (defined here as >65 years as this is usually exclusion criteria in AIT trials) but SLIT with grass pollen and HDM has been demonstrated to be effective and safe in 2 studies.159, 175 AIT elicits clinical responses comparable to studies with younger patients. Another important consideration in this age group, when contemplating treatment with AIT, is the higher prevalence of comorbidities. Examples are hypertension, coronary artery disease, cerebrovascular disease, malignancy and/or cardiac arrhythmias. Also, treatment with medication such as beta-blockers may impair the treatment of anaphylaxis with adrenaline (epinephrine) (see Table 2). AIT can be recommended in otherwise healthy elderly patients with AR whose symptoms cannot be adequately controlled by pharmacotherapy (Grade A for SLIT, B for SCIT).
6.5 Pregnancy
There is 1 prospective study investigating the safety of AIT in pregnancy161 and several retrospective studies that suggest that there is no greater risk of prematurity, fetal abnormality, or other adverse pregnancy outcome in women who receive AIT during pregnancy.39 Observations about anaphylaxis in pregnant and breastfeeding women are largely derived from case reports and are generally reassuring.162 However, the balance between benefits and potential risks in pregnant patients needs to be discussed with the patient. Systemic reactions and their resultant treatment can potentially harm the baby and/or mother. It is therefore recommended that AIT is not initiated during pregnancy (Grade D) but, if already initiated, AIT may be continued during pregnancy or breastfeeding in agreement with the patient's general practitioner (GP) and obstetrician if former AIT treatment has previously been tolerated well (Grade C).
6.6 Adherence
There is a great variance between studies (both real-life studies and clinical trials) in the criteria used for evaluating adherence and in the rates of adherence.163-169 The range of reported adherence varied from 18% to over 90%, higher in clinical studies than real-life surveys with overlapping ranges for SCIT and SLIT. The main causes for poor adherence are reported to be side-effects, inconvenience, lack of efficacy or forgetting to use.163-165, 167, 168, 170 A few other factors have been associated with poor adherence, for example, age and patient's educational level. Potential ways to improve adherence are the use of reminder mechanisms (e.g, alarm on mobile phone, Internet-based tools, short message service (SMS) electronic reminders, social networks, mobile applications (apps), and monitoring systems—this approach should be tailored to the patient (Grade C). Patient education and good communication between physician and patient are key (Grade C).169 One randomized study suggests that adherence is much better with 3-monthly follow-up appointments compared to 6 or 12-monthly follow-up (Grade B).171 Recommendations are summarized in Table 6.
| Recommendation | Evidence level | Grade of recommendation | Strength of recommendation | Other considerations | Key references |
|---|---|---|---|---|---|
| Polysensitized patients | |||||
| Polysensitized patients who are monoallergic are recommended to receive AIT for the specific allergen that is driving their AR symptoms | I | A | Strong recommendation, based on RCTs with low risk of bias56, 109 | Expert review of RCTs137, 149 | Didier56, Demoly137, Durham109, Nelson149 |
| Polysensitized patients who are poly-allergic for taxonomically related homologous allergens can be recommended to receive either a single allergen or a mixture of homologous allergens from that biological family that covers all the major allergens | II | B | Expert review of RCT data | Demoly137, EMA advice132 | |
| Patients who are poly-allergic for nonhomologous allergens may be recommended to start AIT with either the allergen responsible for most of their allergic rhinoconjunctivitis symptoms or separate treatment with the 2 clinically most important allergens | II | C | Expert review of RCT data | Demoly137, EMA advice132, Pfaar142 | |
| Co-existing asthma | |||||
| Controlled asthma is not a contraindication to AIT | I | A | Strong recommendation based on low risk of bias studies43 | Evidence described in asthma AIT systematic review.43 | Dhami14, Virchow41, Dhami43 |
| Specific pediatric issues | |||||
| Consideration of AIT is recommended in pediatric patients with AR with evidence of IgE sensitization to clinically relevant allergens | I | A | Strong recommendations from low risk of bias studies [e.g 90,91,92,98] | See Table 3 for detailed review. | Bufe98, Caffarelli90, Pajno91, Stelmach92 |
| In children aged 2-5 y of age, it may be recommended that consideration should be given to likely benefits and risks associated with AIT for AR | IV | D | Weak recommendation based on little available evidence | May be more difficult to make a definitive diagnosis of AR in preschool children. Safety seems to be similar in this age group as per older patients. | Rienzo173, Rodriguez-Santos174 |
| Elderly | |||||
| AIT can be recommended in otherwise healthy elderly patients (>65 y) with AR | I | A (SLIT), B (SCIT) | Moderate recommendation for SLIT based on 2 consistent RCT studies of unclear risk of bias.159, 175 Moderate recommendation for SCIT based on only one relatively small, low risk of bias study.160 | Detailed clinical assessment is recommended to exclude other types of rhinitis in elderly patients. | Bozek 2012175, 2014159, 2016160 |
| Pregnancy | |||||
| Immunotherapy is not recommended to be initiated during pregnancy | V | D | Based on balance of additional risk vs benefits. | Expert opinion | |
| Maintenance immunotherapy may be recommended to be continued (at the achieved dose) during pregnancy | III | C | Weak recommendation based one cohort study161 and one case series40 | Shaikh161, Metzger40 | |
| Adherence | |||||
| It is recommended that patients should be informed about how immunotherapy works and the need to take regular doses and complete the course of treatment. | IV | C | Based on a survey of allergists. | Based on observational data | Scurati164 |
| Reminders are recommended for patients on immunotherapy to improve treatment adherence. | III | C | One interventional study (educational session, phone calls, emails) | Consider mobile phone texts, social media, and applications (apps) | Savi169 |
| Patients receiving SLIT can be recommended to be followed up every 3 mo to improve treatment adherence | II | B | Moderate recommendation based on one quasi-randomized study.171 | Method of randomization unclear. | Vita171 |
7 HOW LONG AIT SHOULD BE CONTINUED FOR IN AR?
Most clinical studies evaluating the efficacy of AIT follow participants for 1 or 2 years on therapy. The EMA currently recommends an experimental, randomized, controlled design involving 3 years of therapy with a 2-year follow-up period off treatment. These studies demonstrate a sustained benefit for 3 years of SLIT-tablet grass pollen therapy for 2 years off therapy.94, 109, 111, 176 There are some data to suggest that HDM SLIT tablets give sustained benefit for at least 1 year after 1 year of therapy in 1 RCT53 and also after 3 years of therapy in a SLIT drop RCT.177 More data are required for HDM, and evidence is required on the optimal duration of therapy. Grass pollen SCIT for 3-4 years has been shown to result in long-term efficacy for 3 years after discontinuation.83 In a recent study, either SCIT or SLIT tablets were effective compared to placebo over 2 years, but 2 years were insufficient for long-term efficacy as measured 1 year off treatment.65 In another adult study, participants randomized to 3 years of ragweed continued to benefit after 2 years post-SCIT.178 Similarly, children randomized to 3 or 5 years HDM SCIT had similar outcomes at 5 years.179 So, in summary, for patients with AR, a minimum of 3 years of AIT is recommended to achieve long-term efficacy after treatment discontinuation (Grade A) (Table 7).
| Recommendation | Evidence level | Grade of recommendation | Strength of recommendation | Contextual comments | Key references |
|---|---|---|---|---|---|
| AIT is recommended as benefit is seen from the first year of therapy | I | A | Strong recommendation based on low risk of bias studies (e.g53, 56, 58, 69, 72, 74, 85, 94) | Generally consistent data | Dhami14, Bergmann53, Bousquet74, Didier94, Dahl85, Frew58 |
| It is recommended that to achieve long-term benefits, immunotherapy should be continued for a minimum of 3 y | I | A | Strong recommendation based on low risk of bias long-term adult studies,56, 83, 84, 94, 108, 109, 145 one high risk of bias pediatric study (due to its open design although it was randomized)63 plus one recently published low risk of bias pediatric study.111 | Consistent data |
Adult: Arroabarren179, Didier56, Didier108, Didier94, Durham83, Durham109, James84, Lin177, Naclerio178, Ott145, Scadding65 |
8 ADVERSE EVENTS WITH AIT FOR AR
8.1 SCIT
Subcutaneous immunotherapy is a safe and well-tolerated treatment when the injections are given in a medical setting by experienced personnel trained in the early recognition of systemic reactions and how to manage them (Table 8).11, 180-182 There must be immediate access to resuscitation equipment and a physician trained in the management of anaphylaxis (Grade C).
Systemic allergic adverse reactions to SCIT can range between mild-to-severe adverse reactions of the skin, upper and lower airways, gastrointestinal tract, or the cardiovascular system (see Table S2 in online supplement for details of classification).123 In a 3-year real-life US survey study that included over 20 million injection visits, systemic reactions were reported in 0.1% of injections; there were no fatalities182 although 4 were reported in a follow-up survey by the same group.183 Fatal allergic adverse reactions have though been reported in earlier surveys.30, 31 Over 80% of reactions occurred within 30 minutes after injection; very few of the delayed ones were severe. It is therefore recommended that patients stay in clinic for at least 30 minutes after an injection (Grade C).
A European real-life, prospective, survey performed by members of the Immunotherapy Interest Group of EAACI on 4316 patients in France, Germany, and Spain was published after our SR was completed.184, 185 It demonstrated that SCIT and SLIT for respiratory allergy are safe in general in the pediatric and adult population and found only a low number of systematic reactions (SRs). For SCIT, SRs were found in 2.1% of all SCIT-treated patients. Independent risk factors for SRs during SCIT were the use of natural extracts, the absence of symptomatic allergy medications, asthma diagnosis, sensitization to animal dander or pollen, cluster regimens (vs rush), and a previous episode of anaphylaxis. Further possible risk factors for systemic adverse reactions have been described (Box 311). When 1 or more severe adverse reactions occur, the allergist (specialist and subspecialists) should re-evaluate the benefits and risks of SCIT therapy with the patient and decide whether or not treatment should be continued (Grade D). In any case, cessation of treatment or adaptation of the dosing schemes for the next injection should follow the summary of product characteristics (SmPC).
Redness, itching, or swelling represents local reactions at the injection site and occurs frequently after around half of injections.14 Local measures (e.g, cooling or topical glucocorticoids) or oral antihistamines may be helpful for these reactions. Increased local adverse reactions do not predict an increased individual risk of a systemic adverse reaction.186 In case of enlarged local adverse reactions (redness and/or swelling >10 cm in diameter) occur at the injection site, the SmPC provides adaptation of the dosing schemes for the next injection. When local adverse effects occur, premedication with an H1-antihistamine can be used to reduce the frequency and severity of adverse reactions (Grade A recommendation), but this prophylactic treatment does not prevent the onset of SRs or anaphylaxis.187, 188 Also, studies indicate that modified allergen extracts are associated with less adverse effects.189-192 For aluminum hydroxide containing SCIT products, granulomas have been described from a foreign body reaction mainly caused by incorrect intradermal administration as well as contact allergic reactions, new onset of protein contact dermatitis, or a vasculitis inflammatory reactions have been reported.193-195 If these reactions to SCIT occur, treatment with another aluminum hydroxide-free product is preferred (Grade D).11
BOX 3. Risk factors for systemic reactions during AIT
| Current allergy symptoms and potential allergen exposure |
| Current infections |
| Mast cell disease |
| Previous systemic reaction to SCIT or SLIT |
| Uncontrolled or severe asthma |
| A high degree of sensitization |
| Excess dose escalation during initiation |
| Beta-blockers use |
| Poor injection technique |
| Overdose of allergen extract |
| Failure to follow manufacturer's recommendation for dose reduction when change to new production batch |
| High-intensity physical exercise |
8.2 SLIT
Sublingual immunotherapy is regarded to be a safe and well-tolerated treatment (Table 8).11, 14, 196-198
Severe SRs with SLIT appear to be much less likely than with SCIT although the overall rate of any adverse reactions is similar in both SCIT and SLIT14, 184 (see Tables S2 and S3 in online supplement for details of classification123, 199). In a review of 66 SLIT studies (over 4000 patients who received over a million doses), there was 1 SR for approximately every 4 years of treatment and only 1 severe SR per 384 treatment years.198 There are no new safety concerns in more recent studies.14 Several severe reactions—in some cases with anaphylaxis—are described in the literature occurring within 30 minutes of sublingual administration of allergens in droplet or tablet form.34 In these cases, SLIT was not administered according to the standards (nonstandardized extracts, rush protocols, excessive allergen dose, patients in whom SCIT had previously been interrupted due to severe reactions). Patients should be observed for at least 30 minutes after the first dose (Grade C) and supervised by staff able to manage anaphylaxis (Grade C). As in SCIT, concomitant, uncontrolled asthma has been reported to be associated with severe systemic reactions after SLIT.34 In the recently published European Survey, the rate of SRs under SLIT was also reported to be low (1.1% of all SLIT-treated patients).184, 185
The majority of adverse events in SLIT develop at home without any medical observations. Patients should therefore be thoroughly informed about how to recognize and manage reactions, particularly severe ones (Grade D). Patients also need education on what to do if a dose is forgotten and when SLIT should be temporarily interrupted (e.g, oropharyngeal lesions) (Grade D).11 When 1 or more severe adverse reactions occur, the allergist (specialist and subspecialists) should rediscuss the benefits and risks of SLIT with the patient and decide whether or not treatment should be continued (Grade D). As for SCIT, cessation of treatment or adaptation of the dosage should follow the summary of product characteristics (SmPC).
The frequency of local adverse events during SLIT correlates with the dosage and has been reported to be 40-75%, for example, temporary local mucosal reactions (oral pruritus or dysesthesia, swelling of the oral mucosa, throat irritation) or abdominal pain.34, 197-199 Most of these reactions occur during the initial phase of the treatment course (commonly in the first 3 weeks). They are commonly considered to be of mild intensity and self-limiting.34, 97 Nevertheless, these reactions may lead to cessation of treatment, as observed in 4-8% of cases reported in recent trials of SLIT tablets.56, 85, 99, 138(see section “adherence”). As in SCIT, local adverse reactions may be diminished by the intake of oral antihistamines (Grade A).
For SLIT, temporary cessation of therapy may be advised in a number of situations to reduce the potential for adverse effects. For example, for 7 days following dental extraction or oral surgery or following shedding of a deciduous tooth; while an oral ulcer or open wound in the mouth heals; or during an upper respiratory tract infection in patients with asthma. Individual product SmPCs may list additional advice.
| Recommendation | Evidence level | Grade of recommendation | Strength of recommendation | Contextual comments | Key references |
|---|---|---|---|---|---|
| SCIT or SLIT | |||||
| For correctly selected patients, SCIT or SLIT is recommended as, appropriately administered, it is safe and well tolerated | I | A | Strong recommendation based on low risk of bias RCT studies and observational studies14 | Consistent evidence | Dhami14 |
| It is recommended that asthma should be controlled before commencing AIT as insufficiently controlled asthma is a risk factor for both SCIT and SLIT | III | C | Expert opinion from observational studies | Bernstein31, Amin200, Calderon34 | |
| Premedication with an antihistamine is recommended as it reduces the frequency and severity of local and systemic cutaneous reactions but does not eliminate the risk of other systemic adverse reactions including anaphylaxis | I | A | Strong recommendation based on low risk of bias RCTs.187, 188 | Consistent strong evidence from RCT studies | Nielsen187, Reimers188 |
| When one or more severe adverse reactions occur, it may be recommended that the allergist (specialist and subspecialists) should rediscuss the benefits and risks of AIT therapy with the patient and decide whether or not treatment should be continued. This decision and continuation of treatment should be in line with the Summary of Product Characteristics (SmPC). | V | D | Expert opinion from clinical experience | Expert opinion | |
| SCIT | |||||
| It is recommended that patients should remain under observation for at least 30 min after a SCIT injection | III | C | Consistent observational data | Epstein182 | |
| If subcutaneous granulomas develop with aluminum hydroxide containing SCIT products, it may be recommended that a replacement allergen extract that does not contain aluminum hydroxide should be used. | V | D | Expert opinion | Pfaar11 | |
| It is recommended that SCIT should be administered by competent staff with immediate access to resuscitation equipment and a doctor trained in managing anaphylaxis. | III | C | Consistent observational data on adverse effects reported in SR | Dhami14 | |
| SLIT | |||||
| It is recommended that patients should remain under observation for at least 30 min after an initial SLIT dosage | III | C | Expert opinion based on consistent observational data | Calderon34 | |
| It is recommended that initial SLIT dosage should be administered by competent staff with immediate access to resuscitation equipment and a doctor trained in managing anaphylaxis. | IV | C | Consistent observational data on adverse effects reported in SR | Dhami14 | |
| It is recommended that patients receiving SLIT should be informed about how to recognize and manage reactions, particularly severe ones. Patients also need to know what to do if a SLIT preparation is forgotten and when SLIT should be temporarily interrupted (e.g, oropharyngeal lesions). | V | D | Expert opinion from clinical experience | Expert opinion | |
9 PREVENTIVE EFFECTS OF AIT FOR AR
A 3-year course of AIT reduces the likelihood that children and adolescents with allergic rhinitis driven by pollen allergy go on to develop asthma up to 2 years post-AIT.23 There is currently no convincing evidence for a preventive effect of HDM AIT or for prevention of new sensitivities.23 This is further discussed in the EAACI AIT Prevention Guidelines.23
10 PHARMACOECONOMIC ASPECTS OF AIT VERSUS PHARMACOTHERAPY FOR AR
Pharmacoeconomic studies that only analyze costs in monetary units have reported beneficial healthcare expenditure of AIT in the long-run although savings are not expected in the first year. The majority of pharmacoeconomic studies support the viewpoint that AIT gives value for money, with cost-effectiveness within 6 years of treatment initiation.201 Retrospective and prospective observational studies have shown that SCIT and SLIT positively affect healthcare expenditure in pharmacotherapy with a reduction in expenditure of 12% to 80%.202-206 A reduction in medical costs in the AIT vs placebo groups has been repeatedly reported, but these savings did not compensate the costs of AIT.202, 207, 208
In contrast to cost-only studies, cost-effectiveness and cost-utility analysis evaluate the effects of treatment in terms of clinical benefits or health-related quality of life (i.e, quality-adjusted life years [QALYs]). An incremental cost-effectiveness ratio (ICER), which is defined as costs divided by benefits, can be calculated to estimate the costs of a certain gain. Several health economics studies that include cost-effectiveness and cost-utility calculations have demonstrated that SCIT and SLIT are economically advantageous to pharmacotherapy.209-212
Seven studies based on RCT data conducted from a health system perspective and using QALYS as their outcome measure suggests that SLIT and SCIT would be considered cost-effective in this patient population in United Kingdom at the standard National Institute for Health and Care Excellence (NICE) cost-effectiveness threshold of £20 000 (€24 616) per QALY.213-219 The studies comparing SCIT and SLIT have given mixed results and do not allow us to conclude whether either treatment is more cost-effective.220 ICERs for cost evaluations of AIT seem to vary substantially between different health systems suggesting that straightforward conclusions may not be generalizable even across seemingly similar countries.215 Finally, the quality of the studies and the general lack of attention to characterizing uncertainty and handling missing data should be taken into account when interpreting these results.
11 SUMMARY, GAPS IN THE EVIDENCE AND FUTURE PERSPECTIVES
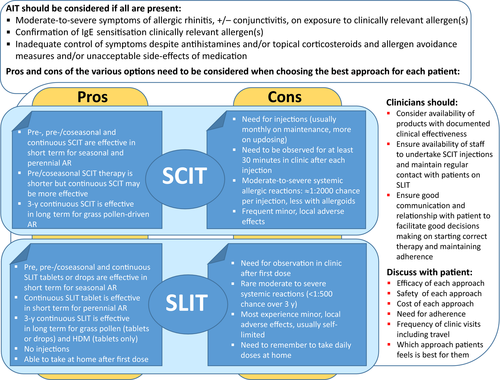
The EAACI Taskforce on AIT for AR has developed this guideline as part of the EAACI AIT Guidelines Project. This guideline has been informed by a formal SR and meta-analysis of AIT for AR.14 The guidelines provide evidence-based recommendations for the use of AIT for patients with AR with or without allergic conjunctivitis. A comparison of SCIT and SLIT is provided in Figure 2. Practical guidance is provided in Box 4 and a summary of the guidelines is provided in Box 5. An approach to the use of AIT for AR across the healthcare system is summarized in Figure 3. The recommendations should be of value to all healthcare professionals involved in the management of patients with AR. There are barriers to the wider use of AIT but equally there are facilitators that could be put into place to widen access to AIT (Table 9).
BOX 4. Practical considerations for healthcare professionals delivering AIT
- Training and facilities
- ○ Expertise in the diagnosis and differential diagnosis of AR by history and supporting SPT or specific IgE testing.
- ○ Training in recognition and management of severe allergic reactions including anaphylaxis.
- ○ Availability of equipment and trained personal to manage severe allergic reactions.
- ○ Training in administration of specific AIT products.
- ○ Facilities to observe patient for at least 30 minutes with SCIT injections and initial dose of SLIT.
- Assessing patient and deciding on best approach
- ○ Effective communication with patients and/or family about practicalities of AIT, expected benefits and potential adverse effects.
- ○ Identification of clinical contraindications to AIT.
- ○ Select an AIT product with documented evidence for efficacy and safety, for the patient's specific presentation, wherever possible.
- Undertaking AIT
- ○ Start AIT for seasonal AR at least 4, and preferably 2, months before the pollen season.
- ○ Preferably start AIT for perennial AR when allergen exposure is lowest and avoidance measures are in place.
- ○ Dose reductions (usually 50%) or split doses for adverse effects, intercurrent illness, or delayed dosing as recommended by SmPC for SCIT.
- ○ Dose interruption with oral lesions and other issues as recommended by SmPC for SLIT.
- ○ Facilities to regularly follow up patient promoting adherences to therapy and watching for adverse effects.
BOX 5. Summary of the EAACI Rhinoconjunctivitis AIT Guidelines
- AIT should be considered with symptoms strongly suggestive of allergic rhinitis, with or without conjunctivitis; evidence of IgE sensitization to 1 or more clinically relevant allergens; and moderate-to-severe symptoms despite regular and/or avoidance strategies.
- AIT may also be considered in less severe AR where a patient wishes to take advantage of its long-term effect on rhinitis and potential to prevent asthma with grass pollen AIT.
- More standardized products with documented evidence for efficacy in clinical trials are needed.
- Standardized AIT products with evidence of efficacy in the clinical documentation should be used when they are available.
- An individual product-based evaluation of the evidence for efficacy is recommended before treatment with a specific product is initiated.
- Key contraindications are severe or uncontrolled asthma; active, systemic autoimmune disorders; active malignant neoplasia. Careful review of benefits and risks is required with history of severe reactions, beta-blocker therapy, severe cardiovascular disease, other autoimmune disorders, severe psychiatric disease, poor adherence, and immunodeficiency. The individual patient's conditions should be considered when deciding whether to prescribe AIT and the summary of product characteristics (SmPC) should be reviewed for specific contraindications for individual preparations.
- For each recommendation, an individual product-based evaluation of the evidence for efficacy is recommended before treatment with a specific product is initiated given the heterogeneity in meta-analysis results:
- ○ Continuous SCIT is recommended for seasonal (Grade A for adults, B for children) or perennial (Grade B for adults, C for children) AR for short-term benefit in those with moderate-to severe disease.
- ○ Pre- and pre-/coseasonal SCIT is recommended for seasonal AR for short-term benefit (Grade A for adults, B for children).
- ○ Both modified (allergoids) and unmodified allergen SCIT extracts are recommended for AR for short-term benefit (Grade A for adults, B for children).
- ○ Continuous grass pollen SCIT is recommended for AR for short- and long-term benefit (Grade A for adults, B for children).
- ○ Pre-/coseasonal or continuous SLIT is recommended for seasonal ARs for short-term benefit (Grade A).
- ○ SLIT with tablets for pollens or HDM can be recommended for AR for short-term benefit (Grade A).
- ○ SLIT aqueous solutions for pollens can be recommended for AR for short-term benefit (Grade B for adults, A in children).
- ○ SLIT aqueous solutions for HDM cannot be recommended for AR for short-term benefit.
- ○ Continuous grass pollen SLIT tablets or SLIT solution is recommended for AR for long-term benefit (Grade A).
- ○ HDM SLIT tablet can be recommended for AR for long-term benefit (Grade B for adults, C for children).
- Polysensitized patients who are poly-allergic for taxonomically related homologous allergens can be recommended to receive either a single allergen or a mixture of homologous allergens from that biological family that covers all the major allergens (Grade A).
- Patients who are poly-allergic for nonhomologous allergens may be recommended to start AIT with either the allergen responsible for most of their allergic rhinoconjunctivitis symptoms or separate treatment with the 2 clinically most important allergens (Grade C).
- In children aged 2-5 y of age, it is recommended that consideration should be given to likely benefits and risks associated with AIT for AR (Grade D).
- AIT can be recommended in otherwise healthy elderly patients with AR whose symptoms cannot be adequately controlled by pharmacotherapy (Grade A for SLIT, B for SCIT).
- If patients have not started AIT and are pregnant, it is recommended to wait until after pregnancy to initiate therapy (Grade D).
- It can be recommended that patients on SLIT are followed up every 3 mo to maximize adherence (Grade B).
- To achieve long-term efficacy, it is recommended that a minimum of 3 y of therapy is used (Grade A).
- Premedication with an antihistamine is recommended as it reduces the frequency and severity of local and systemic cutaneous reactions but does not eliminate the risk of other systemic adverse reactions including anaphylaxis (Grade A).
- It is recommended that patients should wait in the clinic for at least 30 minutes after a SCIT injection (Grade C).
- It is recommended that SCIT should be administered by competent staff, trained to diagnosed symptoms of early systemic reactions or anaphylaxis, with immediate access to resuscitation equipment and a doctor trained in managing anaphylaxis. (Grade C).
- It is recommended that patients should wait in clinic for at least 30 minutes after an initial SLIT dosage and staff and equipment should be available to manage any severe local or systemic reaction or anaphylaxis (Grade C).
- It is recommended that patients receiving SLIT should be informed about how to recognize and manage adverse reactions, particularly severe ones (Grade D).
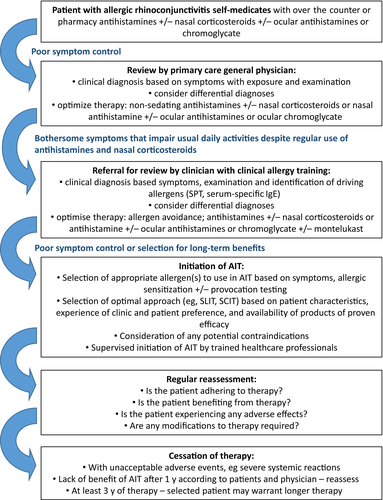
| Recommendation areas | Barriers to implementation | Facilitators to implementation | Audit criteria | Resource implications |
|---|---|---|---|---|
| SCIT or SLIT therapy |
Lack of awareness of how to assess severity of AR Appreciation of SCIT and SLIT as treatment options Access to providers offering SCIT and/or SLIT at convenient locations and/or affordable cost Lack of knowledge about the relative efficacies and safety of different products |
Development of integrated care pathways for AR incorporating primary and secondary care Increase in number of specialists able and willing to provide SCIT and/or SLIT Subsidized provision of SCIT and SLIT Document detailing and training about the efficacy and safety of individual products |
Proportion of patients with moderate-to-severe seasonal AR who are offered and use SCIT or SLIT |
The resource implications include professional time to develop and agree integrated care pathways The costs of training and upskilling allergist (specialist and subspecialists) to deliver SCIT and/or SLIT Training of primary care nurses and doctors to deliver immunotherapy as shared care agreements where appropriate Financial costs of subsidizing access to SCIT and SLIT |
| Selecting the appropriate AIT in patients with polysensitization +/− polyallergy |
Lack of documentation for individual AIT products Effective identification of the key allergen(s) driving symptoms |
Information to clinicians and patients about the better efficacy of single allergen or a mixture of well-documented homologous allergens Use of component-resolved diagnosis and provocation testing |
Proportion of patients receiving either a single allergen or a mixture of well-documented homologous allergens Proportion of patients where additional measures are taken to identify the driving allergen(s) |
Training for clinicians Availability of appropriate AIT products Access to component-resolved diagnostics and provocation testing |
| Using AIT in patients with controlled, co-existing asthma | Lack of education of clinicians and patients |
Information to clinicians and patients about safety of AIT with co-existing asthma Control asthma before commencing AIT |
Proportion of patients with co-existing asthma receiving AIT | Available AIT service |
| Consideration of AIT in pediatric patients with AR | Available AIT clinical service for children | Information about the place of AIT in managing AR in children for health purchases, primary care clinicians and patients | Proportion of pediatric patients with moderate-to-severe seasonal AR who use continuous SCIT | Availability of a clinical service for children able to deliver AIT for AR. |
| Consideration of AIT in otherwise healthy elderly patients with AR | Lack of access to AIT for AR in general or specific products | Information about the place of AIT in managing AR in the elderly for health purchases, primary care clinicians and patients | Proportion of elderly patients with moderate-to-severe seasonal AR who use AIT | Availability of a clinical service able to deliver AIT for AR |
| Adherence to AIT | Lack of patient education about AIT |
Information for patients and use of simple reminders Three-monthly follow-up for SLIT patients Good physician–patient relationship and communication regarding side-effects and time course of treatments |
Assessment of understanding of patients on AIT Assessment of adherence and use of reminders by patients on AIT |
Resources to educate patients Investment in written communication and regular follow-up with access to advice regarding side-effects if necessary |
| Use of premedication with an antihistamine to reduce adverse effects | Lack of knowledge by clinicians and patients | Training of clinicians using AIT | Proportion of patients who receive premedication with antihistamine |
Resources for training clinical staff Availability of medication |
| Observation for at least 30 min after a SCIT injection or initial SLIT dosage by trained staff |
Lack of understanding by clinicians of delayed effects Lack of trained staff and workforce time pressures |
Training of clinicians using SCIT and SLIT Staff availability and rotas for administration and observations |
Proportion of patients who wait 30 min after receiving SCIT or initial SLIT dosage Proportion of staff trained in management of severe adverse reactions |
Resources for training clinical staff Time set aside for observation |
| Information for patients receiving SLIT about how to recognize and manage reactions and when therapy should be temporarily interrupted | Lack of understanding by patients receiving SLIT and clinicians administering | Training of patients and clinicians | Proportion of patients receiving SLIT trained in the self-management of severe adverse reactions | Resources for training patients and clinicians |
The key limitation of this guideline is the considerable heterogeneity seen in elements of the underpinning meta-analysis. For newer products, such as the SLIT grass pollen and house dust mite tablets, we have consistent low risk of bias data and very secure recommendations. For older products, such as house dust mite SCIT products, there is considerable heterogeneity in the meta-analysis weakening the strength of recommendations around those products. Many of these older studies were poorly designed and reported; for example, it is often not clear whether intention-to-treat or per-protocol analyses were being reported making it impossible to combine similar analyses in the meta-analysis. Indirect comparisons within the meta-analysis strongly suggest that some products are more effective than others. A network analysis approach, which allows indirect comparisons across trials based on a common comparator (usually the placebo group), would allow us to improve our comparative estimates between products.221 This would allow product-specific recommendations to be made. The different local regulations47 and availability of products48 makes this difficult at a European level. So before treatment with a specific product is initiated, clinicians need to undertake an individual product-based evaluation of the evidence for efficacy, focusing on low risk of bias studies which are generally the larger, more recent ones.11
There are a number of areas in this guideline where there is no low risk of bias evidence, and these signify the gaps in the current evidence base. The key ones are highlighted here and in Table 10. There is a major gap in the evidence base for the clinical effectiveness of AIT in children and adolescents with recommendations at least 1 grade lower than for adults in most areas. As AR usually starts in childhood and AIT has the potential to change the natural course of the disease and prevent the development of asthma, this age group has most to benefit. Once safety is established in adult studies, pediatric studies need to be commenced using validated, common outcome measures.11 There are also little data in the elderly particularly for patients with multimorbidity. Additionally, more RCTs need to follow participants postcessation of therapy to establish long-term clinically effectiveness, especially for HDM respiratory allergy. Dose-finding studies are needed. Agreement about the clinically meaningful effect size of AIT treatment would assist in the interpretation of clinical trial data and help facilitate stratification studies to help predict which patients will respond best to which forms of AIT. The collection of patent-reported outcomes in studies would ensure the patient experience is captured. Additionally, we need data from randomized cost-effectiveness and cost-utility studies to use in discussions with healthcare funders. We need biomarkers to predict and quantify the effectiveness of AIT to assist in patient selection.222 Suboptimal adherence with AIT is likely to impact on its effectiveness; novel approaches to improve effectiveness should be developed in partnership with patients. Also, to allow better comparison of safety between approaches, studies need to use a unified approach to classifying side-effects is required. A common and international recognized language should be used when reporting severe adverse reactions, such as the MedDRA classification and AIT-related local and systemic reactions should be reported in line with internationally standardized classification such as the WAO-grading system.123, 199 Filling these gaps would allow the generation of much clearer guidelines for clinicians allowing them to stratify patients to the best therapy. It may not be possible to achieve this with only randomized, controlled prospective data; large, real-life, controlled data need to be examined although the potential for bias and confounding needs to be acknowledged.
| Gaps | Plan to address | Priority |
|---|---|---|
| Lack of biomarkers to predict and quantify the effectiveness of AIT | Prospective observational studies to validate potential predictive biomarkers | High |
| Agreement about the clinically meaningful effect size of AIT treatment (active vs placebo treated patients) | Consensus discussion | High |
| Low risk of bias randomized controlled data for children and adolescents | More prospective controlled trials using standardized products | High |
| Evidence for long-term clinical effectiveness after treatment cessation | More prospective controlled trials with follow-up post-treatment cessation in adults and children | High |
| Standardization of grading of adverse effects of AIT | Future clinical trials should use the WAO local and systemic reaction grading system | High |
| Approaches to improve adherence with AIT | Working with patients to develop novel approaches that can be tested in prospective controlled trials and real-life settings | High |
| Randomized cost-effectiveness and cost-utility studies adjusted to socioeconomic differences within and between countries | Additional multinational studies with a health economics focus | High |
| For some AIT products, there is little or no evidence for clinical effectiveness | Dose ranging studies to optimize dose for efficacy and safety; prospective controlled trials; use of patient reported outcomes; use of products with proven effectiveness | High |
| Approaches to minimize adverse effects | More prospective observation and controlled trials. A subanalysis of different phenotypes populations in current RCTs and real-life settings | Moderate |
| Effectiveness of mixtures of homologous allergens from the same, related or different biological families | More prospective controlled trials using the commonest allergens | Moderate |
| Good evidence base for contraindications to AIT | Registries recording patient details, AIT, outcome and adverse effects | Moderate |
| Value of provocation tests in identifying the most appropriate allergen to use in AIT | Prospective controlled studies to assess benefit of provocation testing | Moderate |
| Management of AIT in patients who become pregnant on therapy | More prospective observational studies | Low |
| Lack of standardized AIT preparations for orphan allergens | Multicentre studies | Low |
Despite all these gaps, we have clear evidence for the clinical effectiveness of AIT, for SCIT, SLIT tablets, and SLIT drops, for adults and children with moderate-to-severe AR that is otherwise uncontrolled despite pharmacotherapy. We have evidence-based recommendations for specific patient groups and specific approaches. There is now a need to ensure that primary care healthcare professionals know which patients might benefit from AIT (Box 6), that national healthcare providers understand that AIT is cost-effective and that patients and patient support groups are aware of this approach. This will be supported by the implementation strategy for this guideline with efforts being put into disseminating the guideline. This will be supported with materials such as schedules and country-specific product evaluations as exemplified by the German, Austrian, and Swiss guideline.11 Finally, as new evidence is published, these guidelines will need to be updated with revision of specific recommendations to reflect the new data.
BOX 6. Key messages for primary care
- Diagnosis of AR is by history
- Where severe, treat with nonsedating, long-acting antihistamine and topical nasal corticosteroid (with appropriate nasal spray training) and/or topical ocular cromoglycate or antihistamine.
- Check for any co-existing asthma; this should be properly controlled when using AIT.
- AIT is effective for AR driven by pollens, house dust mite, and animal dander.
- AIT is indicated for AR with moderate-to-severe symptoms that are not controlled by pharmacotherapy or avoidance strategies (where appropriate).
- AIT may be given by subcutaneous (SCIT) or sublingual route (SLIT) as either SLIT tablets or SLIT drops.
- AIT therapy needs to be continued for at least 3 y for postcessation effectiveness.
- Local adverse effects, which are mild in severity and self-limited without the use of rescue medication, are common with SLIT when starting therapy.
- More severe systemic allergic adverse events are infrequently seen and more commonly with SCIT than SLIT.
- SCIT injections and the initial SLIT dose should be given by healthcare personal who are trained in AIT and the management of any adverse events.
- At least a 30-minute observation period is required for all SCIT injections and the initial dose of SLIT.
ACKNOWLEDGMENTS
The EAACI Guideline: AIT for rhinoconjunctivitis Taskforce would like to thank Kate Crowley and Lynn Reeve for their administrative assistance; Stefan Vieths and Andreas Bonertz for their advice; Claus Bachert, G. Walter Canonica, Gabriele Di Lorenzo, Peter Eng, Hans Joergen Malling, and Harold Nelson for their constructive, expert review of the draft guidelines; all the EAACI members who commented on the draft guideline via the public Web site; and to funding from EAACI and the BM4SIT project (grant number 601763) in the European Union's Seventh Framework Programme FP7.
AUTHOR CONTRIBUTIONS
G Roberts and O Pfaar jointly chaired the EAACI Guideline: AIT for rhinoconjunctivitis Taskforce; together with A Muraro and A Sheikh, they conceptualized the manuscript. CA Akdis, IJ Ansotegui, SR Durham, R Gerth van Wijk, S Halken, D Larenas-Linnemann, R Pawankar, C Pitsios, A Sheikh, and M Worm all initially drafted sections of the guideline. S Arasi, M Calderon, C Cingi, S Dhami, JL Fauquert, E Hamelmann, P Hellings, L Jacobsen, EF Knol, SY Lin, P Maggina, R Mösges, H Oude Elberink, G Pajno, EA Pastorello, M Penagos, G Rotiroti, CB Schmidt-Weber, F Timmermans, O Tsilochristou, E-M Varga, J Wilkinson, A Williams and L Zhang as members of the Taskforce plus I Agache, E Angier, M Fernandez-Rivas, M Jutel, S Lau, R van Ree, D Ryan, and GJ Sturm as chairs of the other AIT Guidelines were all involved in conceptualizing the guidelines and critically reviewed guideline drafts. S Dhami and S Arasi also provided methodological support to the Taskforce. F Timmermans was the patient group representative. All the authors satisfied the international authorship criteria (further details in Table S2). This guideline is part of the EAACI Guidelines on AIT, chaired by Antonella Muraro, and coordinated by Graham Roberts.
CONFLICT OF INTEREST
G. Roberts has a patent issued: “Use of sublingual immunotherapy to prevent the development of allergy in at risk infants”; and his university has received payments for the activities he has undertaken giving expert advice to ALK, and presenting at company symposia for ALK, Allergen Therapeutics, and Meda, and serving as a member of an Independent Data Monitoring Committee for Merck outside of this work. O. Pfaar reports grants and personal fees from ALK-Abelló, Allergopharma, Stallergenes Greer, HAL-Allergy Holding B.V./HAL-Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Lofarma, Biotech Tools S.A., Laboratorios LETI/LETI Pharma, and Anergis S.A.; grants from Biomay, Nuvo, and Circassia; and personal fees from MEDA Pharma, Sanofi US Services, Mobile Chamber Experts (a GA2LEN Partner), Novartis Pharma and Pohl-Boskamp, outside this work. CA Akdis has nothing to disclose. IJ. Ansotegui reports personal fees from SANOFI, Bayer, Pfizer, FAES FARMA, MIT FARMA, HIKMA, Menarini, and Bial Aristegui, outside this work. S. Durham reports grants from Regeneron (USA), Biotech Tools, ALK (Denmark), Food Standards Agency (UK), and National Institute of Health Research (UK) and personal fees from Anergis (Switzerland), Circassia (UK), Biomay (Austria), Merck, Allergy Therapeutics (UK), ALK (Hørsholm, Denmark), med update GmbH (Germany), and Allergy Therapeutics, outside of this work. R. Gerth van Wijk reports personal fees from ALK-Abello, Circassia, and Allergopharma, during the conduct of this work. S. Halken reports personal fees from ALK-Abello and from different companies, for example, Meda, Stallergenes, Allergopharma, and ALK-Abello, outside of this work. D. Larenas-Linnemann reports grants and personal fees from Astrazeneca, Boehringer-Ingelheim, MEDA, Novartis, grants and personal fees from Sanofi, UCB, GSK, Pfizer, MSD, grants from Chiesi, TEVA, personal fees from Grunenthal, Amstrong, Stallergenes, ALK-Abelló, personal fees from DBV, outside the submitted work; and Chair immunotherapy committee CMICA, Member immunotherapy committee or interest group EAACI, WAO, SLAAI, Board of Directors and Program Chair CMICA 2018-2019. R. Pawankar has nothing to disclose. C. Pitsios has nothing to disclose. A. Sheikh reports grants from the EAACI during the conduct of this work. M. Worm reports grants from Allergopharma, Novartis, Stallergenes, Medic Pharma, and ALK-Abello. S. Arasi reports payment from Evidence-Based Health Care Ltd during the conduct of this work. M. Calderon has received honorarium in advisory boards for ALK and Hal-Allergy and served as a speaker for ALK, Merck, and Stallergenes Greer. C. Cingi has nothing to disclose. S. Dhami reports grants from EAACI to carry out the review, during the conduct of this work. JL Fauquert has nothing to disclose. E. Hamelmannhas served on scientific advisory boards and received honorarium for lectures on scientific meetings for ALK, AllergoPharma, Bencard, HAL, Leti, Stallergenes. P. Hellings has nothing to disclose. L. Jacobsen reports personal fees from EAMG, outside this work. E.F. Knol has nothing to disclose. S.Y. Lin has nothing to disclose. P. Maggina has nothing to disclose. R. Mosges reports personal fees from ALK, Allergopharma, Allergy Therapeutics, Friulchem, Hexal, Servier, Klosterfrau, Bayer, FAES, GSK, MSD, Johnson& Johnson, Meda, Stada, UCB, and Nuvo; grants from ASIT biotech, Leti, Optima, bitop AG, Hulka, and Ursapharm; grants and personal fees from Bencard and Stallergenes; grants, personal fees, and nonfinancial support from Lofarma; nonfinancial support from Roxall, Atmos, Bionorica, Otonomy, and Ferrero; and personal fees and nonfinancial support from Novartis, outside this work. H. Oude Elberlink reports grants from ALK-Abello during the conduct of this work. G.B. Pajno reports grants from Stallergenes during the conduct of this work. E.A. Pastorello has nothing to disclose. M. Penagos reports personal fees from Stallergenes and ALK, outside this work. G. Rotiroti reports personal fees from ALK-Abello, outside this work. C. Schmidt-Weber reports grants from Allergopharma and Leti and honorarium from PLS-Design, Allergopharma, and Leti; is a member of scientific advisory board for Leti; holds shares in PLS-Design; and hopes to develop a patent. F. Timmermans has nothing to disclose. O. Tsilochristou has nothing to disclose. E-M Varga reports lecture fees from ALK-Abello, Stallergenes Greer, Allergopharma, Bencard, MEDA, and Nutricia outside the submitted work. J. Wilkinson has nothing to disclose. A. Williams reports other grants from ALK-Abello (UK) and Diagenics Ltd (UK), outside this work; and travel expenses for education meetings from the EAACI and BSACI. L. Zhang has nothing to disclose. I. Agache has nothing to disclose. E. Angier reports previous advisory board membership for Stallergenes, Meda, and Schering Plough plus a sponsored lecture by Meda and attendance at a ALK SOSA meeting. M. Fernandez-Rivas reports personal fees from ALK, Merck, and GSK. M. Jutel reports personal fees from Allergopharma, Anergis, Stallergenes, ALK, LETI outside the submitted work. S. Lau reports a grant from Allergopharma plus personal fees for data monitoring committee activities for Merck. R. van Ree reports personal fees from HAL-Allergy BV and Citeq BV outside of the submitted work. D. Ryan reports personal fees from Stallergenes, Thermo Fisher, MEDA outside of the submitted work. G. Sturm reports grants and personal fees from ALK-Abello, Novartis, Stallergenes, Bencard Allergy, and Leti outside of the submitted work. A. Muraro reports speaker's honoraria for Novartis, Meda, Nestle, Stallergenes, ALK outside the submitted work.




