Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study
Summary
Background
Cases of severe drug hypersensitivity, demonstrating a variable spectrum of cutaneous and systemic involvement, are reported under various names, especially drug reaction with eosinophilia and systemic symptoms (DRESS). Case definition and overlap with other severe cutaneous adverse reactions (SCAR) are debated.
Objectives
To analyse the spectrum of signs and symptoms of DRESS and distribution of causative drugs in a large multicentre series.
Patients and methods
RegiSCAR, a multinational registry of SCAR, prospectively enrolled 201 potential cases from 2003 to mid-2009. Using a standardized scoring system, 117 cases were validated as showing probable or definite DRESS.
Results
The male/female ratio was 0·80; females were borderline significantly younger than males. Next to the ubiquitous exanthema, the main features were eosinophilia (95%), visceral involvement (91%), high fever (90%), atypical lymphocytes (67%), mild mucosal involvement (56%) and lymphadenopathy (54%). The reaction was protracted in all but two patients; two patients died during the acute phase. Drug causality was plausible in 88% of cases. Antiepileptic drugs were involved in 35%, allopurinol in 18%, antimicrobial sulfonamides and dapsone in 12% and other antibiotics in 11%. The median time interval after drug intake was 22 days (interquartile range 17–31) for all drugs with (very) probable causality, with differences between drugs.
Conclusion
This prospective observational study supports the hypothesis that DRESS is an original phenotype among SCAR in terms of clinical and biological characteristics, causative drugs, and time relation. The diversity of causative drugs was rather limited, and mortality was lower than that suggested by prior publications.
Drug reaction with eosinophilia and systemic symptoms (DRESS), a rare but potentially life threatening adverse drug reaction (ADR), is characterized by a combination of symptomatic and asymptomatic features, variable both in time and course.1 Commonly reported features are multi-organ involvement, lymphocyte activation (lymph node enlargement, lymphocytosis including ‘atypical’ activated lymphocytes), eosinophilia, reactivation of herpes viruses, and a later onset and longer duration than other cutaneous adverse drug reactions.
The syndrome, first ascribed to aromatic antiepileptic drugs, has been reported under various names, including anticonvulsant hypersensitivity syndrome, drug induced hypersensitivity syndrome (DIHS), DRESS or names referring to the causative drug, the most prominent affected organ or the disease mimicked.2-5 Since the word ‘hypersensitivity’ is rather uninformative and ambiguous, the more informative and clinically relevant acronym DRESS is gaining use.
Besides aromatic antiepileptic drugs, many other drugs have been reported to be associated, such as sulfonamides, allopurinol, minocycline, mexiletine and many other drugs.1, 6-8 The estimated risk at first or second prescription of an aromatic antiepileptic drug is 1–4·5 in 10,000.9 The onset is rather delayed, often 2–8 weeks after the introduction of the inciting drug, although rechallenge can result in a reaction within hours to days.1, 4, 10-13 Recovery after withdrawal of the drug is often complete, but symptoms may persist for weeks to months.12 Mortality rates of about 10% are regularly reported.1, 12-16
DRESS represents a challenging diagnosis, reached after exclusion of other diseases. Diagnosis can be delayed or go unrecognized as drug related because of the variable presentation, course, severity, relatively late onset, gradual evolution and long duration, even after stopping the drug, or because of clinical similarity to infections, collagen vascular or lymphoproliferative diseases.
Knowledge about DRESS mostly relies on case reports and retrospective series, often originating from dermatologists because skin involvement is one of the first and most frequent symptoms observed.1, 2, 4, 15-18 Notwithstanding general agreement about the main characteristics of the syndrome, its definition, clinical and biological features need more accurate appraisal.
Its complete pathogenesis is not yet understood and appears to be complex, combining delayed immunological reactions to drugs, a transient state of immune suppression and reactivation of latent herpes virus infections.5, 19-21 A genetic predisposition was observed in Han Chinese, with a 100% association between DRESS induced by allopurinol and HLA-B*5801.22
Because clear case definition including the cut-off points of abnormal biological and laboratory values was lacking, and in order to obtain a homogeneous cohort of patients for further study, the RegiSCAR study group developed a diagnostic validation score, combining clinical and biological criteria for validating the diagnosis of potential cases of DRESS as definite, probable, possible, or no case (see Appendix S1).23 We present the first large prospective series of 117 cases, collected by the RegiSCAR group, of patients with validated probable or definite DRESS according to this score.
Patients and methods
Setting
RegiSCAR, a multinational registry of severe cutaneous adverse reactions (SCAR), conducts a prospective, ongoing pharmaco-epidemiological study of Stevens Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN), acute generalized exanthematous pustulosis (AGEP), and DRESS, including the collection of biological samples, started in 2003. Through a network of hospitals in Austria, England, France, Germany, Israel, Italy, Taiwan and the Netherlands about 120–150 million inhabitants are covered. The study has been approved by the ethical committee of each participating national centre.
Recruitment of cases
Hospitalized patients, notified to the national investigators and meeting three or more of the inclusion criteria (acute rash, fever above 38 °C, enlarged lymph nodes in at least two sites, involvement of at least one internal organ, or abnormalities in blood count), were consecutively enrolled as potential cases of DRESS.23 After informed consent and using a structured, reaction-specific questionnaire, trained interviewers collected detailed data on the morphology and extent of the rash, clinical and biological data on organ involvement, course of the disease, concomitant/earlier diseases and infections, and exposure to medication.
Validation of cases
Based on clinical photographs, histopathology and completed questionnaires with clinical and biological information, but without information on drug exposure and other risk factors, an international expert committee validated potential cases as definite, probable, possible or no case, following a standardized procedure and scoring system (see Appendix S1).23 Signs and symptoms were only attributed to DRESS after exclusion of alternative causes.
Assessment of drug causality
The evaluation of drug causality was an expert decision by consensus between three authors (SHK, MM, JCR). A first selection, blinded for the name and indication of drugs, was based on the time relation between onset of the reaction and the initiation and withdrawal of each drug. The probable index-day, representing the most likely onset of the reaction, is defined as the earliest date of a clinical sign or symptom consistent with a continuum in the disease. Latency was defined as the number of days between initiation of medication with a probable or very probable causality and the probable index-day. Drugs taken long term (> 3 months), or stopped > 14 days before or initiated < 3 days before the probable index-day, were considered unlikely. Prior use without a cutaneous adverse drug reaction decreased suspicion, while an earlier reaction made the drug a first rank suspect. Hereafter, remaining suspected drugs were unblinded and evaluated according to a list of likelihood for eliciting DRESS, based on a literature review. Causality in cases with a single remaining drug was considered ‘very probable’ in the presence of highly likely medication, and ‘probable’ when medication was not notorious or had low notoriety. For cases with several remaining drugs, those with high notoriety were considered ‘probable’ and those with low notoriety ‘possible’. Causality in cases with concomitantly used drugs without notoriety was classified as undetermined.
Data management and statistical analysis
All collected data were entered by the investigators into a centralized database (Oracle, version 8.1.7 (8i), Redwood Shores, CA, U.S.A.) at the University Medical Center Freiburg, Germany, using the internet-based remote-data-entry system MACRO (version 3.0, InferMed, London, U.K.). For subsequent data processing (including regular data checks, data preparation and data analyses), the software package SAS (version 9.2, Cary, NC, U.S.A.) was used. For statistical analysis, the software package SAS, SPSS (version 16, SPSS, Chicago, IL, U.S.A.), and MS Excel Data Analysis were used. Categorical and dichotomous variables were presented in absolute numbers and percentages, while mean and standard deviation or, if more appropriate, median and interquartile range were presented for continuous variables. For testing differences between groups, we used the nonparametric Wilcoxon test for continuous variables and χ2 test for categorical variables, assuming a two-sided 5% significance level.
Results
Inclusion
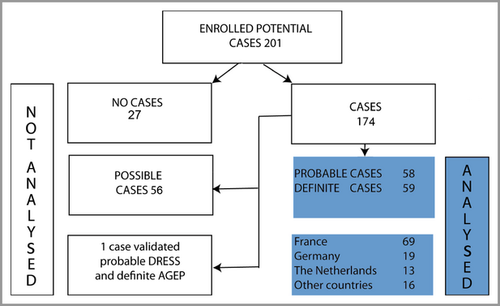
Between February 2003 and May 2009, a total of 201 potential cases of DRESS was included. Of these, 27 were validated as no case, 56 possible, 59 probable and 59 as definite cases of DRESS. With exception of one probable case, also fulfilling the criteria for definite AGEP, all probable and definite cases (n = 117) were analysed in this study (Fig. 1).
Demographics
As shown in Table 1, 96 patients were community cases, admitted because of the reaction, whereas in 21 patients the reaction started in hospital. An earlier cutaneous adverse drug reaction had been experienced by 22 patients, including one with a prior episode of DRESS to the same drug. Females were predominant and borderline significantly younger than males (P = 0·05), especially in those patients whose reaction related to antibiotics (median 39 vs. 51 years), while no difference was observed for allopurinol (median 63 vs. 61 years) and antiepileptics (median 40 vs. 39 years). There was no patent difference by gender in the indication for treatment. The most frequent comorbidity was epilepsy (20·0%). We considered 12 patients (10·3%) to be immunocompromised: one by comorbidity (HIV infection) and 11 by comedication (systemic use of corticosteroids and/or other immunosuppressive or modulating agents).
| Demographics | DRESS n = 117 | SJS/TENa n = 379 |
|---|---|---|
| Sex, male/female ratio | 0·80 (52/65) | 0·62 (145/234) |
| Age all (median, interquartile range) | 48 (30–62) | 50 (28–68) |
| Age male (median, interquartile range) | 56 (34–66) | 47 (30–62) |
| Age female (median, interquartile range) | 44 (29–59) | 51 (28–72) |
| Community cases | 96 (82·1%) | 379 (100%) |
| Earlier cADR | 22 (19·8%) | 52 (13·7%) |
| Comorbidities | ||
| Convulsive disorders | 23 (20·0%) | 47 (12·4%) |
| Collagen vascular disease | 10 (8·5%) | 27 (7·1%) |
| Diabetes mellitus | 14 (12·0%) | 36 (9·5%) |
| Pre-existing kidney disorder | 7 (6·0%) | 30 (7·9%) |
| Pre-existing liver disorder | 6 (5·2%) | 26 (6·9%) |
| Recent cancerb radiation therapy | 6 (5·1%) | 40 (10·6%) |
| 1 (0·9%) | 16 (4·2%) | |
| HIV | 1 (1·3%) | 25 (6·6%) |
| Acute infections (4 weeks before onset of reaction) | 25 (22·9%) | 165 (43·5%) |
| Concomitant medication | ||
| Immunosuppressive or immunomodulating agentsc | ||
| corticosteroids ≤ 8 weeks/> 8 weeks | 7/3 (8·5%) | 14·8% |
| other ≤ 8 weeks/> 8 weeks | 1/0 (0·9%) | |
- cADR, cutaneous adverse drug reaction. aEuroSCAR-study32. bRecent cancer: diagnosed during last 2 years before index date or, if diagnosed earlier, still being treated. cNot including colchicine, combined with allopurinol ≤8 weeks in four cases; four patients using corticosteroids were also using other immunosuppressive or immunomodulating agents.
Characteristics
As shown in Table 2, fever ≥ 38·5 °C was documented in 90% (and fever 38·0–38·5 °C in 7%); 21% experienced more than one episode of fever (≥ 38·0). Lymphadenopathy was observed in 54%.
| n = 117Number | Percentagea | 95% CI | |
|---|---|---|---|
| Fever ≥ 38·5 °C | 103/114 | 90 | 83–95 |
| Lymphadenopathy | 61/112 | 54 | 45–64 |
| Haematological abnormalities | 114/114 | 100 | 97–100 |
| Eosinophilia | 108/114 | 95 | 89–98 |
| grade 2 (≥ 1500 μL−1) | 92 | 81 | |
| grade 1 (700–1499 μL−1) | 16 | 14 | |
| Atypical lymphocytes | 68/102 | 67 | 57–76 |
| Leucocytosis > 10 000 μL−1 | 99/104 | 95 | 89–98 |
| Neutrophilia > 7000 μL−1 | 81/104 | 78 | 69–85 |
| Lymphocytosis > 4000 μL−1 | 54/104 | 52 | 42–62 |
| Monocytosis > 1000 μL−1 | 47/68 | 69 | 57–80 |
| Thrombocytosis > 400 000 μL−1 | 20/107 | 19 | 12–27 |
| Thrombocytopenia < 100 000 μL−1 | 7/107 | 7 | 3–13 |
| Skinb | 117/117 | 100 | 97–100 |
| Extent of rash > 50% | 79/100 | 79 | 70–87 |
| Suggestive rash | 68/100 | 68 | 58–77 |
| Facial oedema | 89/117 | 76 | 67–83 |
| Monomorphic maculopapular | 18/117 | 15 | |
| Polymorphous maculopapular | 99/117 | 85 | |
| Urticarial | 10 | 9 | |
| Exfoliative | 11 | 9 | |
| Lichenoid | 4 | 3 | |
| Pustules | 35 | 30 | |
| Purpura | 31 | 26 | |
| Infiltrated plaques | 27 | 23 | |
| Blisters | 19 | 16 | |
| Target–like lesions | 14 | 12 | |
| Eczema–like lesions | 8 | 7 | |
| Duration exanthema ≥ 15 days | 55/60 | 92 | 82–97 |
| Mucosal involvement | 66/117 | 56 | 47–66 |
| Mouth/throat/lips | 61 | 52 | |
| Eyes | 15 | 13 | |
| Genitalia | 8 | 7 | |
| Other | 8 | 7 | |
| Internal organ involvement | 107/117 | 91 | 85–96 |
| 1 organ involved | 42 | 36 | |
| 2 organs involved | 41 | 35 | |
| > 2 organs involved | 24 | 21 | |
| Liver | 86/114 | 75 | |
| Kidney | 40/108 | 37 | |
| Lung | 33/104 | 32 | |
| Muscle/heart | 13/99 | 13 | |
| Spleen | 12/79 | 15 | |
| Pancreas | 3/77 | 4 | |
| Otherc | 13/117 | 11 | |
| Duration DRESS ≥ 15 days | 107/109 | 98 | 94–100 |
- aDenominator number of cases, investigated for the feature. bAvailable pictures 106 (91%) and biopsies 91 (78%). cIncluding gastro-intestinal tract (6) central nervous system (5), thyroid gland (2).
All cases (100%) showed one or more haematological abnormality. Eosinophilia, defined as an absolute eosinophil count ≥ 700 μL−1, was present in 95% and ≥ 1500 μL−1 in 81%. Atypical lymphocytes were observed in 68 cases (67%). Although not part of the validation score, we also noticed other haematological abnormalities. Leucocytosis [median 19 000 μL−1, interquartile range (IQR) 15 800–29 100 μL−1] was found in 95% and lymphocytosis in 52%. Neutrophilia (78%) was predominantly present in the early phase of the reaction, while monocytosis (69%) occurred later. Lymphopenia (5%, data not shown), thrombocytopenia (7%), and thrombocytosis (19%) were infrequent.
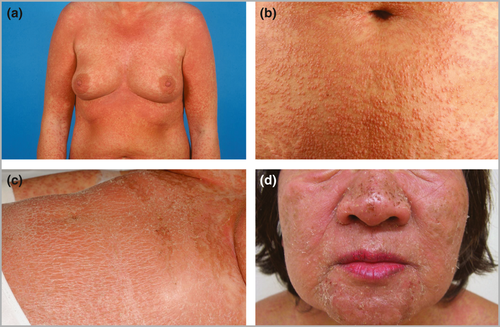
All patients experienced an acute skin eruption (Fig. 2). The extent and morphology however were only rated when photographs were available (n = 106) and informative (n = 100). In 79% the skin involvement exceeded 50% of the body surface area, while in 68% morphology fulfilled two or more criteria suggesting DRESS (see Appendix S1). The rash was a monomorphic maculopapular, sometimes confluent and/or oedematous erythema in 15%, while in all other cases it was polymorphous, including additional varying combinations of other lesions such as pustules or tense blisters with, except for two cases, negligible detachment. Pruritus (81%) was more frequent than burning or pain (35%). Facial oedema was observed in 76%. Mild mucosal involvement was recorded in 56%; in 15% more than one mucous membrane was affected. Most frequent were oral lesions (52% of all patients), including the lips (25%), oral cavity (40%) and throat (8%).
Two or more internal organs were involved in 56% and one in 36%. Most frequently the reaction affected the liver (75%), kidney (37%) and lung (32%). Kidney involvement was significantly more frequent in cases related to allopurinol than in those to carbamazepine (60% vs. 17%, P < 0·01), whereas liver involvement did not differ significantly.
HHV6 reactivation was demonstrated in serum samples in 21 of 58 patients routinely investigated in the active phase of the disease (36%) by polymerase chain reaction and/or positive IgM or a fourfold rise in IgG titre. EBV/CMV reactivation was observed in three patients.
Course and outcome
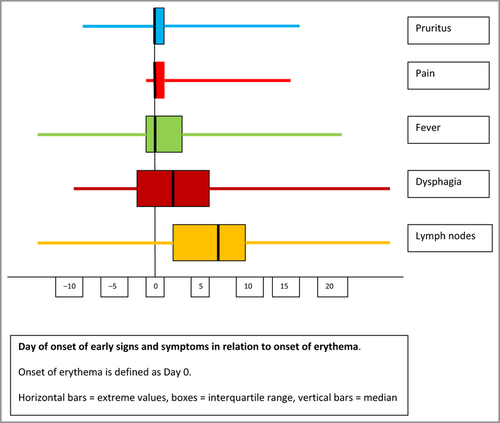
Some signs or symptoms such as pruritus, skin pain, fever, dysphagia and lymphadenopathy may appear before skin lesions (Fig. 3). In 92% the skin eruption persisted ≥ 15 days, while in all but two the full course of DRESS lasted ≥ 15 days. The median inpatient stay for community patients was 17 days (IQR 12–25 days). During the acute phase, 2 of 117 patients died.
Causative drugs
The results of the expert decision on drug causality are presented in Table 3. Overall 634 medicines (median 4, IQR 2–7 per patient), containing 316 different therapeutically active components, were used in the month before the probable index-day. The number of suspect drugs was substantially reduced after elimination as a result of the time course. In 66 cases, only one (43) or two (23) drugs remained, most often including a single drug of high notoriety. All patients in whom highly notorious drugs were eliminated because of long term use (allopurinol 61, 102 and 132 months, lamotrigine 9 months, oxcarbazepine 11 months, phenobarbital 20 months, and fluindione 408 months) were exposed to an alternative highly notorious drug within the chosen time window.
| Exposure | Cases | Median | Interquartile range |
|---|---|---|---|
| At least one drug | 115 (98%) | ||
| Total number of drugs used | 634 | 4 | 2–7 |
| Causality | Cases | Drugs | |
| Very probable | 39 (33%) | 39 | |
| Probable | 54 (46%) | 57a | |
| Possible | 10 (9%) | ||
| Undetermined | 5 (4%) | ||
| Unlikely | 7 (6%) | ||
| No drug use | 2 (2%) | ||
| Associated very probable drugs | Median latency | Interquartile range | |
| AED | 41 (35%)b | ||
| Carbamazepine | 23 | 29 | 20–36 |
| Phenytoin | 8 | 29 | 27–37 |
| Lamotrigine | 8 | 27 | 20–34 |
| Oxcarbazepine | 2 | n.a. | n.a. |
| Phenobarbital | 2 | n.a. | n.a. |
| Allopurinol | 21 (18%) | 20 | 17–30 |
| Sulfonamides | 14 (12%) | ||
| Sulfasalazine | 8 | 20 | 18–25 |
| Dapsone | 3 | n.a. | n.a |
| Sulfamethoxazole–trimethoprim | 2 | n.a. | n.a |
| Sulfadiazine | 1 | n.a. | n.a. |
| Antibiotics | 13 (11%) | ||
| Vancomycin | 7 | 17 | 13–21 |
| Minocyclin | 4 | 20 | 17–26 |
| Amoxicillin | 1 | n.a. | n.a. |
| Ampicillin/sulbactam | 1 | n.a. | n.a. |
| Other drugsc | 5 (4%) | 26 | 25–28 |
- n.a., not applicable, AED, aromatic antiepileptic drugs. aEqually suspected highly notorious drugs in the same case: allopurinol/fluindione, oxcarbazepine/phenobarbital, and carbamazepine/phenytoin. bTwo equally suspected aromatic antiepileptic drugs in two cases. cFlavoxate, fluindione, nevirapine, phenylephedrine-acetaminophen, strontium ranelate.
Aromatic antiepileptic drugs were considered responsible in 35%. Epilepsy was the most common indication for the use of phenytoin or lamotrigine, while carbamazepine was prescribed for other indications, e.g. mood disorders or pain, in 61%. An aromatic antiepileptic drug (phenytoin) was once combined with dexamethasone for seizure prophylaxis in brain tumour. Allopurinol (18%), was prescribed for gout in six and hyperuricemia in 15 patients, with a starting daily dose of 300 mg in 15 patients, 200 mg in two, and 100 mg in four patients. Antimicrobial sulfonamides or dapsone (sulfas) were suspect in 12%, especially sulfasalazine and dapsone, other antibiotics in 11%, predominantly vancomycin and minocycline, and other drugs in 4%. In 9%, drugs of low notoriety were concomitantly used with drugs without notoriety, while another 4% were classified as undetermined, because of polypharmacy and the absence of a notorious drug. No likely drug could be detected in 6%. Two patients (2%) were not exposed to any drug in relevant period.
For all cases with a ‘very probable’ or ‘probable’ causality, the median latency was 22 days (IQR 17–31 days). Between the two most often observed drugs, carbamazepine and allopurinol, latency did not differ significantly (P = 0·11).
Overlap with other types of severe cutaneous adverse reaction
Of 118 patients with probable or definite DRESS, eight shared some features with SJS or TEN or AGEP and were re-reviewed for the alternative SCAR. Three cases were considered a true overlap: one patient with probable DRESS fulfilled the criteria of definite AGEP and was discarded from the analysis in this study, one patient with definite DRESS was also validated as having probable AGEP (simultaneous onset), and one patient with definite DRESS as having probable SJS/TEN (onset 3 days after the index date for DRESS); both were included as DRESS in the present study.
Discussion
Strengths and weaknesses of the study
This large observational multinational study allows detailed analysis of the clinical and biological features of DRESS and the relative contribution of high risk drugs. Since notification and enrolment of cases was independent of outcome, exposure to specific medication or other risk factors, we consider our results as probably less biased than those of most previous series.15, 16, 24-29 Moreover, the prospective collection of data through direct interviews of patients and treating physicians, using a structured reaction-specific questionnaire, and validation by an expert committee using a standardized scoring system, clinical photographs, clinical and biological parameters contributes to the strength of the study. Our validation criteria and cut-off points are stricter than those of earlier case series, resulting in less heterogeneity of cases (see Appendix S1).16, 23, 29 Moreover, evaluation of case reports is often not possible because transparency of data is lacking.18 Combining a scoring system with an expert review, blinded for drug exposure and other risk factors, has proven to be effective for validation in SJS/TEN, and AGEP.30, 31
On the other hand, the nature of our study implies limitations in adequately addressing questions such as the efficacy of treatment, the support of causality by in vivo or in vitro testing of suspected culprit drugs, or late complications and sequelae. Cutaneous manifestations were part of our inclusion criteria, implying that potential cases without overt skin lesions were probably missed. However, a recent review of the literature indicated that almost all reported cases experienced a skin reaction.18
Not all physicians are familiar with DRESS, which introduces the risk of biased notification and absence of relevant laboratory investigations during the first days of the reaction, and potentially resulting in underscoring of asymptomatic features. Moreover, recognition of the syndrome and the complete collection of its symptomatic and asymptomatic features is often complicated. The first symptoms may be seemingly harmless and each feature may be of variable onset and severity, leading to confusion and delay in diagnosis. Furthermore, features like eosinophilia and internal organ involvement tend to abate or disappear after treatment with systemic corticosteroids.
Several findings have been mentioned in previous reports and could be expected since they were part of our diagnostic score. However, the prospective and structured nature of our study enabled a more detailed description of mucocutaneous involvement, and a better assessment of the prodromal period, the prevalence of key features such as eosinophilia and organ involvement, and the distribution of inciting drugs.
Demographics
Contrary to most reports, our study showed a slight female predominance (male/female ratio 0·80).5, 7, 10, 13, 15, 18, 24, 25 This predominance has also been reported for SJS/TEN (0·62), and AGEP (0·80) in a comparable population.32, 33 Women were just significantly younger than men, a difference not observed in SJS/TEN or AGEP (EuroSCAR, unpublished data). We do not have a satisfactory explanation for this finding.
As shown in Table 1, the frequency of previous rheumatic or collagen vascular disease was strikingly high (8·5%) as previously reported for SJS/TEN, while that of cancer (5·1% vs. 10·6%) was lower than for SJS/TEN and close to the control group for SJS/TEN.32 Our collection also included fewer HIV infected patients than observed in SJS/TEN or earlier reported in DRESS.24, 25, 29, 32 This may reflect a different or changing medication profile and also that abacavir hypersensitivity does not sufficiently meet our criteria for DRESS (Appendix S1).23
Contrary to SJS/TEN and earlier case series in DRESS, immunocompromised patients were not clearly overrepresented, and their profile did not significantly differ from that of other cases in our study (data not shown).25, 29, 32 Exposure to corticosteroids appeared to be lower in cases of DRESS (8·5%) than in SJS/TEN (14·8%). This may be related to the relatively high prevalence of corticosteroid maintenance therapy in the context of brain tumour and comedication with aromatic antiepileptic drugs in SJS/TEN.32
Characteristics
High fever usually started at the beginning of the reaction, and regularly preceded the eruption, generating concern about underlying infections.1, 4 A long lasting, polymorphous rash, and facial oedema were characteristic for DRESS. Facial oedema (76%) was more frequently manifest than is often stated.4, 15, 16, 25 Especially in combination with high fever and an eruption, it constitutes a warning signal because in common cutaneous adverse drug reactions the face is usually spared. Mucosal involvement (56%), mainly of the lips and oral cavity, was more frequent than generally assumed; however, in comparison with the findings in SJS/TEN it was rather mild and less haemorrhagic.
Haematological abnormalities were far more frequent and diverse than is usually described, reflecting that retrospectively collected cases are prone to missing informative biological data and underlining the importance of a full blood count in DRESS. In our study, leucocytosis was common (95%) and often considerable. Transient eosinophilia (95%) was far more often present than is usually reported.7, 15, 16, 24, 25, 34 Hypereosinophilia > 1500 cells μL−1, if persistent, can be toxic to endothelial cells and contribute to organ damage such as interstitial nephritis, pneumonitis, myositis, eosinophilic carditis, pancreatitis, thyroiditis or encephalitis.4, 35, 36 Remarkably also, neutrophilia (78%), and monocytosis (69%), usually not reported in DRESS, were frequent. Neutrophils, especially when activated, may also be implicated in tissue damage. Atypical lymphocytes, often regarded as characteristic for DRESS, were found in 67%, while lymphocytosis was observed in 52%. The pattern of leucocytosis, combined with neutrophilia in the early stage and monocytosis in a later stage is frequent in other strong inflammatory reactions; the combination with eosinophilia however is rather characteristic for DRESS. Thrombocytopenia or thrombocytosis, both occasionally reported, were quite infrequent.
Lymphadenopathy (54%) was observed more frequently than is sometimes reported.16, 24, 25, 29 Visceral involvement often determines severity in DRESS. Liver involvement was frequent (75%), most often expressed by transiently disturbed liver function tests, although hepatomegaly, sometimes with coagulopathy, was also observed. In addition, we regularly noted involvement of the kidneys (37%), ranging from mild proteinuria to severe renal function disturbances needing transient haemodialysis (data not shown), lung (32%), and more incidentally of muscle/heart, spleen, pancreas, gastrointestinal tract, central nervous system and thyroid gland.
Reactivation of herpes viruses, especially HHV6, often described in DRESS and even considered a criterion for DIHS by Japanese experts, is held responsible for a more severe and/or protracted course.5, 7, 19, 21, 34, 37, 38 HHV6 reactivation was observed in 21 of 58 of our cases (36%), routinely investigated by the treating physicians. This is less frequent than was reported in earlier, especially Asian series, where it may reach 60%.7, 21 This discrepancy might be explained by fewer elaborate investigations in our cases.
Course and outcome
For the assessment of course, latency time and drug causality, the correct establishment of the onset of the reaction is essential. The prodromal stage, defined as the period between the onset of the reaction and the start of the exanthem, lasted up to 2 weeks (median 1 day, IQR 0–4, range 0–14 days). Early signs and symptoms such as fever, lymphadenopathy, flu-like symptoms, sore throat or dysphagia, burning pain and pruritus can easily be overlooked or misdiagnosed. Incidentally, we also noticed asymptomatic organ or haematological involvement before the onset of erythema. However, laboratory investigations or medical imaging will generally only be performed at a later stage, after admission or the suspicion of DRESS. Notwithstanding our efforts to collect systematically early signs and symptoms, as these are crucial for determining the index date, we cannot exclude that these were sometimes missed, and that the onset of the disease was occasionally a few days earlier. This might partially contribute to the longer latency time in DRESS compared to other SCAR.
Mortality was considerably lower in the acute phase than is usually reported, probably reflecting bias in published retrospective studies.
Causal drugs
The process of determining drug causality was quite straightforward, and very probably causality was more frequent than in SJS/TEN (79% vs. 69%).39 Selection of potential culprits, based on the temporal relation but blinded for drug names, clearly pointed towards notorious suspects in the majority of cases. Noteworthy is the rather limited spectrum of causative drugs in DRESS, with carbamazepine as the leading drug followed by allopurinol. Although lack of controls and validated rules for causality assessment could easily have led to spurious temporal associations of widely used drugs, these did not appear.
Although latency times exceeding 3 months have been reported, risk was mostly confined to medication started within 2 months. Patients in whom highly notorious drugs were discarded for causality because of long-term use had all been exposed to another highly notorious drug at a later date. Risk, confined to relatively recently introduced exposure has also been reported for SJS/TEN and is of considerable relevance for the long-term use of medication such as allopurinol or aromatic antiepileptic drugs.40 In addition, most cases using drugs without significant risk had been exposed to another highly notorious drug at the same time.
As could be expected, epilepsy (20%) was a frequent indication for aromatic antiepileptic drugs in DRESS, although carbamazepine was prescribed in 61% of users for other indications than epilepsy, reflecting its increasing use for new indications. Allopurinol seems to have surpassed sulfas as a frequent inducer of DRESS.11 It was seen as first use except for one patient in whom prior exposure also resulted in DRESS. Noteworthy was the significantly higher prevalence of renal involvement in cases related to allopurinol compared with carbamazepine, whereas earlier reported differences in liver involvement were not found.15, 24, 41 Allopurinol is increasingly prescribed, also without clear indication and at relatively high doses; 17 of 21 patients started allopurinol with a daily dose of ≥ 200 mg. Daily doses equal to or exceeding 200 mg were also associated with a higher risk for SJS/TEN.42
The prodromal stage and the quite prolonged latency time in DRESS introduces the risk of protopathic bias, especially for antibiotics and NSAIDs, necessitating scrupulous investigation of the prodromal stage. There was a tendency to a longer latency for carbamazepine (median 29 days) than for allopurinol (median 20 days). Also remarkable are the shorter latency times in this series compared with earlier series.7, 15, 16, 24 Responsible could be a stricter case definition, leading to a less heterogeneous study population, prospective assessment with more complete collection of data, different drug profiles and more attention to the prodromal period.
Overlap
SJS/TEN and AGEP share some features of internal involvement with DRESS, although to a milder and more limited extent.43 The mucocutaneous features, however, are quite discriminative in all three types of SCAR. Blisters, occasionally present in DRESS, are generally tense and related to dermal oedema.4 Mucosal involvement differs from SJS/TEN in being rather mild. Compared to AGEP, flexural accentuation is lacking, while pustules, if present, are follicular and mainly limited to the face and upper thorax. Also striking is the subacute character and protracted course in DRESS, while AGEP in particular is characterized by an acute onset and quick resolution. The histopathology of DRESS is quite distinct from that of TEN and AGEP and lacks full thickness necrosis or sterile nonfollicular subcorneal pustules.44, 45 Applying the RegiSCAR validation score systems for SJS/TEN and AGEP to patients, validated as probable or definite DRESS, resulted in a negligible overlap.23, 30, 31 This supports the hypothesis that DRESS is an original phenotype and confirms the reliable performance of our scoring systems.
Interestingly, several major culprit drugs are the same in DRESS and SJS/TEN, such as allopurinol, carbamazepine, phenytoin, lamotrigine, and sulfasalazine. In a comparable study-population, overall latency in SJS/TEN and AGEP were shorter than in DRESS.32, 33 Comparing latency in DRESS and SJS/TEN for the two most prevalent drugs in our study, we noticed a significant difference for carbamazepine (median 29 days versus 15 days) while for allopurinol (median 20 days versus 19 days) there was no difference (EuroSCAR unpublished data).
On the other hand, aromatic antiepileptic drugs and allopurinol, important culprit drugs both in SJS/TEN and DRESS, constitute no clear risk in AGEP. Quinolones and aminopenicillins, important triggers for both SJS/TEN and AGEP, are not clearly associated with DRESS, while vancomycin and minocycline, less prominent in other SCAR, are regularly implicated in DRESS. Co-trimoxazole and oxicam NSAIDs, showing a strong association with SJS/TEN, seem a smaller risk factor in DRESS.7, 15, 25, 32, 46
Conclusion
Our validation scoring system for DRESS, based on clinical and biological parameters and the exclusion of other entities, resulted in only minor overlap with other SCAR. Clinical and biological characteristics, causative drugs and the time relation support that DRESS is an original phenotype among the spectrum of ADRs.
DRESS is a serious, multiorgan ADR, exhibiting variable combinations of features. Because cutaneous symptoms are generally present and are often the first and most visible manifestation, the syndrome is classified as a SCAR. However, potentially severe involvement of visceral organs makes this syndrome of interest for all physicians.
Awareness of DRESS is a prerequisite for diagnosis, since it is a syndrome in which signs and symptoms often evolve sequentially. This introduces the risk of delayed diagnosis and separate treatment of each symptom. In particular a high and spiking fever and haematological abnormalities may raise suspicion of an infection. Early recognition, followed by prompt withdrawal of the culprit drug is the most decisive step to avoid disease progression and restore health.
Acknowledgments
We are indebted to all SCAR-patients, other members of the RegiSCAR group, interviewers for the RegiSCAR study, and all the collaborating hospitals and referring physicians for their important contribution to this work.




