The anterior pituitary hormone PRL was identified in animal species as early as 19331 but only purified in humans in 1972.2 Since then, the clinical syndrome of hyperprolactinaemia has been characterized extensively, the predominant symptoms being galactorrhoea, oligomenorrhoea or amenorrhoea and infertility in women and reduced libido, impotence and galactorrhoea in men.3–8 Hyperprolactinaemia has an estimated prevalence of 15% in women with secondary amenorrhoea,9,10 a condition that affects at least 3% of women of reproductive age.11
Pathological hyperprolactinaemia results from a lactotroph adenoma, conditions that increase TRH, such as hypothyroidism, conditions that decrease dopamine action at the lactotroph cell, such as hypothalamic or pituitary tumours, drugs such as dopamine D2 receptor antagonists, or conditions in which reduced clearance of PRL occurs, such as renal failure. By contrast, macroprolactinaemia, the presence of elevated levels of PRL of high molecular mass with little, if any, bioactivity, remains a largely under-recognized phenomenon and is not considered in the differential diagnosis of hyperprolactinaemia in current comprehensive endocrinology texts.3,4,6 Recent studies have indicated that macroprolactinaemia accounts for up to 26% of biochemical hyperprolactinaemia depending on the immunoassay in use,12–18 and thus macroprolactinaemia represents a common diagnostic pitfall, which is responsible for frequent misdiagnosis and mismanagement of hyperprolactinaemic patients.19–23
Prolactin and macroprolactin
Prolactin, a globular protein consisting of 199 amino acids with three intramolecular disulfide bonds, is synthesized as a prehormone with a molecular weight of 26 kDa.24 When the prehormone is proteolytically cleaved, the resulting mature polypeptide has a molecular weight of 23 kDa, and this monomeric form accounts for the majority of total PRL in the serum of normal subjects and most patients with hyperprolactinaemia. Prolactin is secreted episodically by the anterior pituitary and is primarily under tonic inhibitory control of the hypothalamus.25 Physiological levels of PRL are higher during pregnancy and lactation than otherwise and mean serum levels are higher in women than in men.26,27 In addition to monomeric PRL, which accounts for approximately 85% of the total circulating PRL in the majority of normal subjects and in those patients with hyperprolactinaemia, other molecular weight variants of PRL can be demonstrated in serum.28,29 Big PRL, which has a molecular mass in the 50 kDa range and is thought to be a covalently bound dimer of PRL, accounts for approximately 10–15%. Big big PRL, or macroprolactin, which has a molecular mass of more than 150 kDa, usually contributes a small, though variable amount to circulating levels.28,30 Moreover, post-translational modification of pituitary PRL generates a variety of additional species, including glycosylated and phosphorylated variants, together with 14, 16 and 22 kDa proteolysed forms.25
In 1974 Rogol and Rosen31 reported for the first time that big PRL may be the predominant form of PRL in the serum of some patients with hyperprolactinaemia, while in 1981 Whittaker et al. observed that fertility was maintained even when circulating levels of big big PRL were significantly elevated. Numerous subsequent case reports and case series have demonstrated that high levels of PRL, predominantly composed of macroprolactin, are compatible with preserved menstrual cyclicity and ovulation.15,32–37
Nature of macroprolactin
Following the simultaneous demonstrations by Hattori et al.36,37 and Leite et al.38 that the sera of a significant number of patients with idiopathic hyperprolactinaemia contained an anti-PRL antibody, it has become clear that in most cases macroprolactin is a macromolecular complex of 23 kDa monomeric PRL and an immunoglobulin. This high molecular mass form of PRL, despite being present at supraphysiological levels, does not elicit the common signs and symptoms of the hyperprolactinaemic syndrome.34,39,40 Less commonly, other forms of macroprolactin have been described, often in patients with prolactinomas. Such forms are heterogeneous with molecular mass ranging up to approximately 500 kDa. They are incompletely characterized but are often composed of either covalent or noncovalent polymers of monomeric PRL, although some hyperglysolated variants have also been described.24,41–46
Although direct biochemical or structural evidence on the nature of macroprolactin is lacking, there is a considerable amount of indirect evidence that is consistent with the concept that macroprolactin is a PRL autoantibody complex. Such evidence includes the results of immunoadsorption and precipitation experiments using anti-human immunoglobulin G (IgG), protein A and protein G,38,47–49 together with Western blot anlysis.47,50 The molecular mass of macroprolactin, 150–200 kDa on gel filtration chromatography (GFC), is also consistent with the concept of a PRL–immunoglobulin complex. Scatchard analysis by Hattori et al.37 demonstrated that a low affinity (10−6 l/mol) but high capacity (2·1 mg/l) antibody of the IgG subclass directed against PRL was present in the sera of macroprolactinaemic patients. However, evidence by Bonhoff et al.47 suggests that the PRL autoantibody complex is of high affinity. Antibody titres in such individuals were shown to remain relatively constant over a number of years. Recent clinical experience has revealed that macroprolactin in the form of a PRL–autoantibody complex is much more frequently encountered in hyperprolactinaemic sera than any of the other high molecular mass forms.51,52 Consequently, this review focuses primarily on that form of macroprolactin that behaves as a PRL–autoantibody complex.
Experiments that examined the disappearance of human macroprolactin injected into rats suggested that reduced clearance of the high molecular mass complex was likely to account for the persistent hyperprolactinaemia observed in patients harbouring this PRL–autoantibody complex.39 Ahlquist and Fahie-Wilson53 examined the compartmental distribution of macroprolactin in a patient with hyperprolactinaemia and a coexisting pituitary tumour. They observed that while monomeric PRL was identified by GFC in serum, cerebrospinal fluid (CSF) and a pituitary extract, macroprolactin was present only in serum. These results indicate that macroprolactin is formed subsequent to the release of 23 kDa PRL from the pituitary. Furthermore, they demonstrated that the macroprolactin complex is confined to the intravascular space and is not present in the CSF, presumably because of its high molecular weight.
Response to pharmacological stimuli
Intravenous administration of TRH or the dopamine antagonist metoclopramide (MCP) to normal subjects is followed by an increase of approximately 1000–2000 mU/l in serum PRL levels, which peak within half an hour of administration.26 A blunted response is characteristic of patients with true hyperprolactinaemia.26 By contrast, in most macroprolactinaemic subjects the maximal response to these stimuli appears to be normal.38 The largest study to address this was carried out by Vallette-Kasic et al.,54 who reported a normal response of PRL to TRH in 63% and a normal response to MCP in 88% in a series of more than 100 macroprolactinaemic patients. Studies that have examined in greater detail the response to TRH or MCP have identified different kinetic profiles in macroprolactinaemics compared to normal subjects. In macroprolactinaemic subjects, an initial normal peak response, probably caused by release of pituitary monomeric PRL, is followed by a more delayed increase in macroprolactin levels, which has been explained by the binding of monomeric PRL to circulating anti-PRL autoantibodies together with continued PRL secretion.35 Bjøro et al.55 demonstrated that following TRH stimulation, an increase in macroprolactin to levels above baseline could still be demonstrated 24 h following TRH administration. These observations provide convincing evidence of delayed clearance from the circulation of macroprolactin. Similar conclusions are supported by the different rates of decline of monomeric PRL and macroprolactin following administration of bromocriptine. While bromocriptine rapidly suppressed monomeric PRL by approximately 80% after 6 h, a fall of only 20% occurred in macroprolactin during the same time,35 although suppressed values are eventually achieved. These findings in humans are supported by animal studies by Carlson et al.44 and Hattori and Inagaki,39 who observed delayed clearance rates for macroprolactin injected into rats relative to monomeric PRL.
Measurement
Reactivity of macroprolactin in PRL immunoassays
It is not surprising that PRL immunoassays detect macroprolactin. However, what is surprising and of ongoing concern to both clinicians and laboratory scientists is the significant variability in the detection of macroprolactin in hyperprolactinaemic sera by different PRL immunoassays in routine use.56–59 In a comprehensive study, Smith et al.18 examined the ability of nine of the most commonly used immunoassay platforms to measure PRL in 10 sera containing predominantly macroprolactin. The results demonstrated gross variability in the detection of PRL across the nine assay systems with 2·3–7·8-fold differences in measured PRL levels in the 10 sera (Fig. 1). Moreover, comparison of monomeric PRL levels in the 10 sera measured following GFC with the reported PRL levels following immunoassay revealed that all of the automated immunoassay systems detected macroprolactin to some extent. Although the absolute PRL levels varied in the 10 sera examined, there was consistent stratification so that the hierarchy of results obtained was reproduced in each given assay. For example, the Roche Elecsys and Wallac Delfia assays exhibited high reactivity towards macroprolactin while the Bayer Centaur and Beckman Access systems demonstrated low reactivity, with other assays falling in between these extremes.
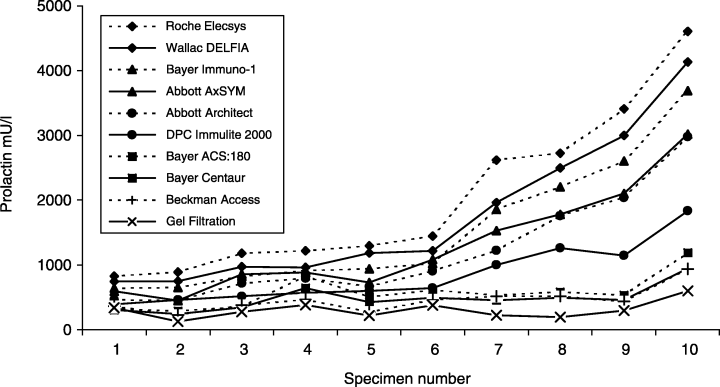
Mean serum PRL levels reported by nine different immunoanalyser user groups in specimens collected from 10 macroprolactinaemic subjects. For comparative purposes the PRL level in each specimen following removal of macroprolactin by gel filtration chromatography is shown. Figure reproduced from Journal of Clinical Endocrinology and Metabolism 2002, 87, 5410–5415. Copyright © 2002, The Endocrine Society.18
The relatively consistent hierarchy in immunoassay reactivity observed between the 10 specimens examined in the study by Smith et al.18 suggests a common endogenous anti-PRL autoantibody directed against a single epitope on PRL. If this is indeed the case, then the variability of PRL immunoassays to detect macroprolactin probably reflects differing specificities in the capture or detection immunoassay antibodies used by the various diagnostic systems. It is likely that the proximity of the epitopes to which the immunoassay capture or detection antibodies are directed, relative to the epitope to which the endogenous autoantibody is directed, may explain the differences in reactivity of the various commercial immunoassays.
Gel filtration chromatography (GFC)
A variety of approaches have been used to determine the level of macroprolactin in serum. Traditionally, GFC has been used to fractionate the various isoforms of PRL including macroprolactin in serum.28,35,60,61 Typical elution profiles obtained on chromatography of normal and macroprolactinaemic sera are illustrated in Fig. 2. Results have been generally expressed as percentage of PRL present in the high molecular weight or macroprolactin form. For example, macroprolactin accounts for 85% of PRL in the macroprolactinaemic serum illustrated in Fig. 2. However, it is also possible to provide the absolute quantity of macroprolactin present. Conventionally, a diagnosis of macroprolactinaemia has been attributed to hyperprolactinaemic patients when more than 30–60% of PRL was in the macroprolactin form.13–15
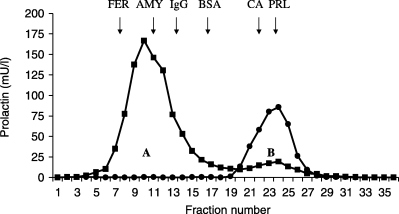
Gel filtration profiles obtained following chromatography of serum from a macroprolactinaemic patient (▪) on Sepharose G-200 and a true hyperprolactinaemic patient (•) for comparison.23 The two discrete peaks of PRL immunoreactivity, representing macroprolactin (peak A) and monomeric PRL (peak B) are present. Arrows indicate the elution positions of molecular weight markers: apoferritin (FER) 443 kDa; β-amylase (AMY) 200 kDa; human immunoglobulin G (IgG) 150 kDa; bovine serum albumin (BSA) 66 kDa; carbonic anhydrase (CA) 29 kDa; monomeric prolactin (PRL) 23 kDa.
Although the technique of GFC is robust and reproducible and is often considered the ‘gold standard’, it does suffer from four main disadvantages. First, with a low affinity antibody complex as reported by Hattori et al.,37 there exists the potential for dissociation of PRL from the autoantibody during the lengthy gel filtration run, thereby leading to an underestimation of the macroprolactin content in serum. In practice, dissociation to any appreciable extent does not seem to take place, indicating that macroprolactin is probably a high affinity complex. Second, there is considerable inherent cumulative imprecision associated with measuring the levels of PRL and macroprolactin in 30–40 discrete fractions to obtain an estimate of the percentage of macroprolactin present. Third, procedural loss of PRL immunoreactive material through adsorption or denaturation during the gel filtration run if selective, that is a disproportionate loss of either PRL or macroprolactin, would lead to either under- or overestimation of the individual isoforms present. Fourth, the labour-intensive nature and expense of GFC usually preclude its widespread use in all but research laboratories.
Polyethylene glycol (PEG) treatment
Polyethylene glycol (PEG) at a concentration of 12·5% (w/v) has the ability to precipitate immune complexes and this method has been widely used to screen for the presence of macroprolactin in hyperprolactinaemic serum since described by Hattori et al. in 1992. 36,37 The method has been validated against GFC by a number of groups14,15,62 and PRL recoveries of < 40% following treatment of sera with PEG have been proposed for the detection of macroprolactinaemia. While there is a relatively good correlation between GFC and PEG precipitation,14,62 quantitatively macroprolactin levels differ. Following precipitation of sera with PEG, macroprolactin levels are significantly higher than values derived following GFC. Suliman et al.23 demonstrated that normoprolactinaemic sera contained between 2% and 9% macroprolactin as determined by GFC, while the same sera treated with PEG yielded an apparent macroprolactin content of between 30% and 36%. Polyethylene glycol-induced coprecipitation of a significant amount of monomeric PRL, together with the small amount of macroprolactin present in normal sera, is likely to account for the discrepancy.
The PEG precipitation method is reproducible, easily performed and is the method of choice for most laboratories.23 However, using relative percentage rather than absolute PRL thresholds renders the result subject to misinterpretation. For example, recoveries of < 40% may be consistent with true hyperprolactinaemia, that is the simultaneous presence of an excess amount of macroprolactin and of supraphysiological levels of monomeric PRL.63 Olukoga and Kane15 reported three patients to have macroprolactinaemia on the basis of PRL recoveries of < 40% although residual monomeric PRL levels ranged from 1500 to 2000 mU/l. Such patients clearly have an excess of both macroprolactin and monomeric PRL. From a clinical point of view, the presence of excess monomeric PRL is of overriding concern and a diagnosis of macroprolactinaemia in this setting is misleading and inappropriate. We have recently proposed a more rigorous definition for the diagnosis of macroprolactinaemia.23 This requires that monomeric PRL levels in hyperprolactinaemic sera fall to within a normal range following removal of macroprolactin. Normative PRL data were derived using PEG-treated sera from 110 healthy women. Prolactin levels fell from 78 to 564 mU/l in untreated sera to 70–403 mU/l in PEG-treated normoprolactinaemic sera. Consequently, for a diagnosis of macroprolactinaemia to be made it is necessary that PRL levels in hyperprolactinaemic sera treated with PEG should fall to those levels obtained when normoprolactinaemic sera was treated similarly, that is to less than 403 mU/l. Application of an appropriate reference range furthermore controls for the coprecipitation of monomeric PRL when serum is treated with PEG. Irrespective of the method used and conventions adopted, it is incumbent on each laboratory to establish an appropriate reference range. Unfortunately, the PEG precipitation method is not applicable to all PRL immunoassays systems for methodological reasons. On some immunoassay platforms, such as Abbott AxSym, PEG is incompatible with the immunoassay format.20,64 In other systems, PEG causes no interference (Delfia), positive interference to varying degrees (Immuno-1, Elecsys and Immulite), or variable negative interference (ACS 180) as assessed by recovery of the PRL standard.13,65–67 Manufacturers of PRL immunoassays have been slow to incorporate interference data, validated protocols or guidelines for laboratories planning to undertake macroprolactin screening using PEG in their assay literature. Clearly, this issue needs to be addressed urgently with the establishment of methods for macroprolactin estimation that have national regulatory body approval. Similarly, it is incumbent on laboratories reporting serum PRL levels to make clinicians aware of their assay characteristics and limitations.68
Immunoprecipitation and adsorption
In 1992, two groups independently demonstrated that both anti-human immunoglobulin and protein A had the ability to precipitate macroprolactin, thereby providing strong evidence that macroprolactin constituted a PRL–IgG complex.37,38 Protein A, a cell wall product of Staphyloccus aureus, has the ability to bind the Fc region of immunoglobulin molecules without interfering with the antigen-binding site. Recently, protein A Sepharose has been used to screen for the presence of macroprolactin by specifically removing PRL–IgG complexes from hyperprolactinaemic sera prior to immunoassay.69 Use of protein G Sepharose, which is somewhat more specific for human IgG, has yielded similar findings to those obtained with protein A.39,49 Smith et al.63 have also examined the ability of both protein A and protein G to remove macroprolactin from sera containing predominantly macroprolactin prior to immunoassay. Results indicated that both of these reagents are effective in removing macroprolactin from serum, although monomeric PRL levels obtained in treated sera were approximately 30% higher than those obtained by GFC. Nevertheless, both protein A and protein G yielded satisfactory correlation coefficients of 0·91 and 0·93, respectively, when compared to GFC.
Ultrafiltration
Ultrafiltration relies on the molecular mass selectivity of specific membranes for plasma proteins. Applying this technique to sera containing macroprolactin, Fahie-Wilson and Heys, 70 Craddock et al.64 and Quinn et al.71 found the procedure to be a useful alternative to PEG precipitation. However, when Prazeres et al.72 compared macroprolactin levels determined by ultrafiltration and GFC, they observed that the data were widely discrepant in a significant number of cases. Similar findings have been reported by this group.73
Overview
From both a clinical and a biochemical perspective, the overriding concern should be to identify those patients with true hyperprolactinaemia correctly in the first instance so as to avoid subsequent unnecessary investigation and treatment. Currently, the only means of screening hyperprolactinaemic sera is to re-assay such sera for PRL after macroprolactin depletion. Of the methods that remove macroprolactin from serum prior to immunoassay, treatment with PEG is the most commonly used procedure.
Measurement or screening for macroprolactin is only necessary where hyperprolactinaemia is detected in the first instance. Using immunoassays that react strongly with macroprolactin, for each 100 hyperprolactinaemic samples screened for macroprolactin approximately 25 will be positive, i.e. 25%. Using a low reacting assay system, of the original 100 hyperprolactinaemic samples as detected in a high reacting assay system, only 80 will be hyperprolactinaemic. When these 80 samples are screened, generally less than five will be positive for macroprolactin, i.e. less than 6·25%.
Bioactivity
In vivo bioactivity
The overall clinical body of evidence is highly suggestive of significantly reduced or limited bioavailability and biological activity of macroprolactin in vivo. When over 10 000 healthy subjects were screened, macroprolactinaemia was found in 0·4% of women, all of whom were free of symptoms.74 However, interpretation of the relationship between macroprolactinaemia and clinical features of hyperprolactinaemia is confounded by the circumstances in which macroprolactin has been assessed. In all of the larger series of macroprolactinaemic patients studied, symptoms characteristic of hyperprolactinaemia, such as oligomenorrhoea or amenorrhoea, galactorrhoea or infertility, have been observed in a proportion of cases.13,15,16,23,38,67,75 However, as measurement of PRL is prompted by symptoms of hyperprolactinaemia and these symptoms are not specific, it is not surprising that occasionally the occurrence of the symptoms and macroprolactinaemia are coincidentally but not causally related. Some studies also reported on hyperprolactinaemic patients in whom investigation for macroprolactin was carried out because of atypical clinical symptoms.33,35,38,76,77 Furthermore, the observation that some macroprolactinaemic patients apparently respond clinically to dopamine agonist (DA) treatment does not necessarily imply that the patient had previously been exposed to supraphysiological levels of bioactive PRL because spontaneous resolution of oligomenorrhoea may occur. It is usual that normoprolactinaemic ‘idiopathic’ galactorrhoea responds to DA treatment, suggesting heightened mammary tissue sensitivity to PRL or the requirement for a permissive level of PRL for galactorrhoea to occur.78,79 However, the frequency of the occurrence of macroprolactinaemia in symptomatic patients may be greater than expected by coincidence.13
In vitro bioactivity
Using an in vitro bioassay developed by Tanaka et al.,80 whereby the mitogenic responsiveness of Nb2 rat lymphoma cells to exogenous lactogenic hormones is assessed, several investigators have examined the bioactivity of macroprolactin. Results of such studies are conflicting, with groups reporting increased, normal or reduced PRL activity in patients with macroprolactinaemia.38,39,52,76,81 The one unifying feature of such studies is that macroprolactin has always been reported to be bioactive to some extent in vitro. Part of the explanation for the above discrepant findings may be related to differences in the PRL preparations tested, with some investigators examining whole sera from macroprolactinaemic patients while others have tested purified or partially purified macroprolactin preparations isolated by immunoaffinity or GFC. Given that macroprolactin is a large molecular mass complex of monomeric PRL and an IgG molecule that appears to be confined to the vascular compartment, PRL is likely to be bio-unavailable in vivo rather than inherently bio-inactive.
Epidemiology and natural history
Prevalence in the general population
In a series of 10 550 healthy Japanese adults (8450 men and 2100 women), 0·4% (40 subjects) had hyperprolactinaemia. A quarter of these hyperprolactinaemic subjects were macroprolactinaemic, eight of whom were women.74 None of these 10 subjects had symptoms or signs of endocrine dysfunction. One other study from Norway, consisting of 660 healthy individuals (280 males), identified only one female patient with macroprolactinaemia.12 These findings suggest that macroprolactinaemia probably exists in the general population at a prevalence of 0·2% in women, but only 0·02% in men.
Prevalence of macroprolactinaemia in the hyperprolactinaemic population
The proportion of hyperprolactinaemic sera explicable by macroprolactinaemia varies depending upon the assay used to measure PRL and on the subgroup of patients studied. Table 1 summarizes the reported prevalence of macroprolactinaemia in studies in which all hyperprolactinaemic samples were screened for macroprolactin. While a lower incidence of 10% was reported by Valette-Kasic et al.,54 two-thirds of the subjects in that study were not screened as they were assumed to have true hyperprolactinaemia based on characteristic clinical findings, and thus it is likely that that figure represents a significant underestimate. By contrast, the highest reported incidence of macroprolactinaemia is 46% of 113 patients studied by Hauache et al.75 The authors acknowledged that the particularly high incidence of macroprolactin in their study probably reflected selection bias because of the specialized nature of the study centre, which received samples sent from other laboratories, when the possible diagnosis of macroprolactinaemia was raised.
| Reference | N | Threshold (mU/l) | Prevalence (%) |
|---|---|---|---|
| Bjoro et al. 199512 | 605 | 1000 | 25 |
| Fahie-Wilson and Soule 199713 | 69 | 700 | 25 |
| Viera et al. 199814 | 1220 | 540 | 36 |
| Olukoga and Kane 199993 | 188 | 430 | 15 |
| Leslie et al. 200116 | 1225 | 700 | 26 |
| Smith et al. 200218 | 300 | 700 | 24 |
| Hauache et al. 200275 | 113 | 620 | 46 |
| Strachan et al. 200367 | 273 | 700 | 21 |
- N, number of subjects with hyperprolactinaemia; Threshold, level of total PRL above which screening for macroprolactin was undertaken.
Intraindividual variation and familial association
In a subset of 42 macroprolactinaemic patients studied by Vallette-Kasic et al.,54 mean PRL levels remained constant during 2–7 years of follow-up, although wide intraindividual variations were seen. Leite et al.,38 however, found a high percentage of macroprolactin in three of 29 first-degree relatives of macroprolactinaemic patients. In view of the very low prevalence of macroprolactinaemia in the general population, these findings are indicative of a genuine, but weak, familial association.
Macroprolactin in pregnancy
When sera from two pregnant females with macroprolactinaemia were examined at frequent intervals throughout pregnancy, serum PRL was initially primarily in the macroprolactin form.82 Furthermore, cord blood obtained at birth was shown to contain appreciable quantities of macroprolactin. Ahlquist and Fahie-Wilson53 have reported similar findings in a neonate, and speculated that transplacental transfer of the anti-PRL–IgG complex from the mother to the baby may be responsible for the phenomenon. Similar to the nonpregnant state, macromolecular forms of PRL distinct from the more common PRL–IgG autoantibody complex have been reported to occur in pregnancy.83
Pascoe-Lira et al.49 studied the presence of anti-PRL antibodies in the sera of 209 healthy women at different stages of pregnancy. PEG precipitation revealed macroprolactinaemia in eight of 209 (3·8%) women and anti-PRL antibodies were found in five of the eight women. Thus, there is some evidence that macroprolactinaemia may be more prevalent during pregnancy than in the nonpregnant state. Furthermore, failure to appreciate that macroprolactinaemia during pregnancy leads to unusually high levels of PRL has led to diagnostic confusion. At least one case report has described inappropriate investigation, including two magnetic resonance imaging (MRI) scans, in a woman who subsequently was found to have macroprolactinaemia.84
Macroprolactin in men and in children
Hyperprolactinaemia in men may be associated with reduced testosterone, decreased libido, galactorrhoea and impotence. These symptoms are at least partially due to suppression of the hypothalamic–pituitary–gonadal axis, although a direct effect of PRL may also occur. Few reports exist concerning macroprolactinaemia in male subjects, but those that exist suggest reduced in vivo bioactivity. Guay et al.85 reported six male subjects who were evaluated for sexual dysfunction and found to have elevated PRL levels but normal testosterone levels and no evidence of any abnormality of the pituitary on MRI scanning. When submitted to GFC, serum PRL from these subjects proved to be predominantly macroprolactin. A surprising feature of this study was that six subjects with macroprolactinaemia were identified from a total of 326 consecutive patients with impotence that were screened. This represents a prevalence of 2%, or approximately 100 times that expected for men in the general population.
The same group subsequently reported on two men with pituitary macroadenomas in whom the majority of circulating PRL was macroprolactin.86 These men had normal sexual function and normal tests of nocturnal penile tumescence and rigidity. However, both had extremely high levels of circulating PRL (> 10 000 mU/l) and there was evidence that both tumours secreted PRL. These findings can be explained by the concept that antibodies directed against PRL may have reduced the bioavailability of PRL and thus the clinical manifestations of hyperprolactinaemia. Hyperprolactinaemia has been described infrequently in childhood, which may reflect the less common measurement of PRL in children. However, there has been a series of four children with macroprolactinaemia reported.87 Two of the children had three computed tomography (CT) or MRI scans each in search of pituitary lesions. No abnormalities were detected. Fideleff et al.88 reported five patients (one male and four females) aged 11–18 years with incidentally discovered asymptomatic hyperprolactinaemia who underwent repeated evaluations over a period of 3 months to 8 years. In all of these cases increased levels of either macroprolactin or big PRL were identified.
Clinical presentation and management of macroprolactinaemia in women
Presentation
In contrast to the consistent clinical findings in hyperprolactinaemia associated with elevated levels of monomeric PRL as occurs in the case of a prolactinoma,89 the reported clinical features of macroprolactinaemia have varied greatly in different studies, reflecting the circumstances that prompted the measurement of PRL (Table 2). Case reports and series in which macroprolactin was measured because clinical features were not typical of hyperprolactinaemia have concluded, not surprisingly, that clinical features of macroprolactinaemia differ from those of true hyperprolactinaemia. This observation was first made by Whittaker et al.,81 who reported maintained fertility in a woman with hyperprolactinaemia predominantly accounted for by macroprolactin. Subsequently, Andino et al.33 reviewed six regularly ovulating women with hyperprolactinaemia and reported that in all of these patients macroprolactin was the principal form of PRL in sera. Since then, numerous reports have documented macroprolactinaemia in patients who exhibit biochemical hyperprolactinaemia but lack the clinical features of hyperprolactinaemia.15,32,35–37,90–92 However, it is not surprising that symptoms and signs of hyperprolactinaemia have frequently been found to coexist with macroprolactinaemia as it is these symptoms that prompt initial measurement of PRL (Table 2). Leite et al.38 identified macroprolactinaemia in 11 hyperprolactinaemic subjects, seven of whom, 64%, had presented with galactorrhoea, menstrual disturbance or both. Vallette-Kasic et al.54 studied 106 macroprolactinaemic patients, 96 of whom were adult females. In this cohort of patients, screening for macroprolactin was frequently prompted by atypical features of hyperprolactinaemia, in some cases lack of symptoms; hence, a selection bias probably existed. However, galactorrhoea was observed in 46% of subjects and menstrual disturbance in 39%. Following exclusion of women who were perimenopausal or had features of polycystic ovary syndrome (PCOS), a total of 25% had menstrual disorders. Hauache et al.75 observed that while symptoms were more likely to occur in subjects with true hyperprolactineamia (90%), they were also frequently found in macroprolactinaemic subjects (54%).
| Reference | Country of origin | N | Study design | Screening method | Clinical features (%) | Abnormal CT/MRI (%) | DA treatment (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| G | O/A | SUB | |||||||
| Leite et al. 199238 | Canada | 11 | P | GFC | 36 | 27 | NR | NR | NR |
| Fahie-Wilson and Soule 199713 | UK | 16 | R | GFC + PEG | 0 | 38 | 6 | NR | NR |
| Olukoga and Kane 199993 | UK | 15 | R/U | PEG | 13 | 80 | 40 | 20 | 73 |
| Leslie et al. 200116 | UK | 55 | P/U | PEG | 2 | 15 | 9 | 7 | 22 |
| Valette-Kasic et al. 200254 | France | 96 | R/S | GFC | 46 | 39 | 29 | 22 | 47 |
| Hauache et al. 200274 | Brazil | 52 | R/S | GFC ± PEG | 25 | 36 | 7 | 21 | 32 |
| Suliman et al. 200323 | Ireland | 21 | R/U | PEG | 29 | 57 | 29 | 15 | 87 |
| Strachan et al. 200367 | UK | 44 | R/U | PEG | 14 | 20 | 11 | 19 | 27 |
- N, number of adult female subjects with macroprolactinaemia; R, retrospective; P, prospective; U, unselected; S, selected; GFC, gel filtration chromatography; PEG, polyethylene glycol precipitation; G, galactorrhoea; O, oligomenorrhoea; A, amenorrhoea; SUB, subfertility, patients presenting who wished to become pregnant; NR, not reported; DA, dopamine agonist.
In contrast to the findings of these studies, two studies have suggested that features of hyperprolactinaemia are relatively uncommon in macroprolactinaemic subjects. Fahie-Wilson and Soule13 reviewed 16 female macroprolactinaemic patients and found two cases of menstrual disturbance that could not be attributed to coexisting PCOS and no instance of galactorrhoea. Leslie et al.16 reviewed 55 macroprolactinaemic patients, and found symptoms of hyperprolactinaemia in only a small number of subjects. The low incidence of clinical features of hyperprolactinaemia in the latter study probably reflects local practices with a low threshold for measuring PRL. The reported indications for measurement of PRL included fatigue, menopausal symptoms and menorrhagia. Classical symptoms of oligomenorrhoea or galactorrhoea were the reason for PRL measurement in only 20% of these subjects.
Management of hyperprolactinaemic patients with macroprolactin
In order to gain insights into the consequences of failure to recognize macroprolactinaemia in hyperprolactinaemic patients, it is necessary to perform retrospective comparative analysis of the management of unselected hyperprolactinaemic patients who were subsequently determined to have either true hyperprolactinaemia or macroprolactinaemia following re-assay of the relevant archived sera. Only two studies meet these criteria. While the patients presented by Leslie et al.16 were unselected, the study was prospective and patients were managed with the information that they had macroprolactinaemia or true hyperprolactinaemia. Both the studies of Hauache et al.75 and Valette-Kasic et al.54 reported findings in hyperprolactinaemic patients specifically selected when clinical features raised the suspicion of macroprolactinaemia.
Olukoga and Kane93 and Suliman et al.23 examined archived hyperprolactinaemic sera retrospectively to identify patients with true hyperprolactinaemia or macroprolactinaemia (Table 2). The medical records of these two cohorts of patients were also examined. Table 3 outlines the findings of Suliman et al.,23 demonstrating that while oligomenorrhoea or amenorrhoea and galactorrhoea occurred more frequently in patients with true hyperprolactinaemia, 84% and 63%, these symptoms also occurred in 57% and 29%, respectively, of macroprolactinaemic patients. These differences, though statistically significant, are clearly not sufficient to distinguish between the two groups of patients on clinical grounds. Consistent with these findings, Olukoga and Kane93 found symptoms potentially attributable to hyperprolactinaemia in all of 17 patients who were retrospectively identified to have macroprolactinaemia. In view of this frequently occurring combination of symptoms and apparent biochemical confirmation of hyperprolactinaemia, it is not surprising that investigation and treatment had been undertaken that may appear to be unnecessary but only with the retrospective knowledge that these patients had macroprolactinaemia. Consistent with this is the report that at least one patient has undergone unnecessary pituitary surgery.15 This group reported that a 31-year-old woman was found to have hyperprolactinaemia during investigation for hirsutism. CT scanning revealed a small pituitary adenoma that was resected transsphenoidally. Postoperatively, there was no change in the patient's symptoms and similarly hyperprolactinaemia persisted. Fifteen years later she was found to have macroprolactinaemia. Dopamine agonist treatment was prescribed for 13 of the 15 retrospectively identified macroprolactinaemic patients by Suliman et al.23 and for 13 of 17 macroprolactinaemic patients reported by Olukoga and Kane.93
| Characteristics | Hyperprolactinaemia (n = 42) | Macroprolactinaemia (n = 21) | Reference interval |
|---|---|---|---|
| Age (years) | 30 ± 1 | 28 ± 3 | |
| Total PRL (mU/l) | 2096 ± 233 | 1524 ± 202 | 78–564 |
| PRL after PEG precipitation (mU/l) | 1705 ± 190 | 202 ± 27* | 70–403 |
| FSH (IU/l) | 5·7 ± 0·5 | 7·1 ± 2·1 | 2–25 |
| LH (IU/l) | 5·3 ± 0·5 | 10·1 ± 2·4* | 2–50 |
| Oestradiol (pmol/l) | 162 ± 33 | 284 ± 48* | 110–1470 |
| Clinical features (%) | |||
| Oligomenorrhoea or amenorrhoea | 84 | 57* | |
| Galactorrhoea | 63 | 29* | |
| Infertility | 8 | 29 | |
| Headache | 8 | 10 | |
| CT/MRI performed (%) | 90 | 93† | |
| Abnormality identified (%) | 34 | 15 | |
| Dopamine agonist prescribed (%) | 88 | 87† | |
- * P < 0·05 vs. true hyperprolactinaemic subjects.
- † Investigations and treatment carried out in subjects in whom macroprolactin was measured retrospectively (n = 15).
Hormonal findings
Oligomenorrhoea or amenorrhoea in the setting of true hyperprolactinaemia is characterized by low levels of oestradiol and low or inappropriately normal concentrations of FSH and LH, reflecting the hypothalamic origin of the disturbance. In the retrospective study of Suliman et al.,23 plasma levels of oestradiol and LH proved significantly higher in macroprolactinaemic compared with true hyperprolactinaemic subjects, consistent with limited bioactivity of macroprolactin (Table 3).
Imaging
The prevalence of abnormal MRI or CT scans performed ranged from 7% to 22% in the six macroprolactinaemic cohorts in which it was assessed (Table 2). However, CT/MRI imaging in the general population, undertaken for reasons other than suspected pituitary disease, indicate that 10–20% of such images are consistent with the presence of a pituitary adenoma.94 Autopsy findings are similar. Therefore, the prevalence of CT/MRI findings consistent with a pituitary adenoma in macroprolactinaemic subjects is similar to that in the general population. Given that macroprolactinaemia can account for up to 26% of hyperprolactinaemia and up to 10% of a population may harbour an adenoma, it is to be expected that some patients will be identified who have both macroprolactinaemia and imaging findings consistent with a pituitary adenoma.92
Re-evaluation of patients retrospectively identified to have macroprolactinaemia
Retrospectively identified macroprolactinaemic subjects whose symptoms had previously been attributed to hyperprolactinaemia require re-evaluation. With the knowledge that macroprolactin accounted for hyperprolactinaemia, revised diagnoses may be possible for some patients. For example, in the series of retrospectively studied patients examined by Suliman et al.,23 one patient with a revised diagnosis of infertility secondary to PCOS was successfully treated with clomiphene and another infertile patient with previously unidentified tubal damage was successfully treated with surgery. In addition, previously prescribed DA treatment should be stopped and the patient re-evaluated.
Response to treatment
Suliman et al.,23 in a retrospective study of unselected patients, noted that most patients with macroprolactinaemia and galactorrhoea, but not those with macroprolactinaemia and menstrual abnormalities, exhibited symptomatic improvement during DA treatment. This is consistent with the beneficial effect of DA treatment on galactorrhoea in normoprolactinaemic subjects and can be explained by the dependence of mammary tissue on permissive circulating levels of PRL for lactation to occur.78 The fall in PRL levels to within the normal range observed in macroprolactinaemic patients on treatment with dopamine agonists may also mislead.
Routine screening for macroprolactin in hyperprolactinaemic sera
Despite convincing evidence of the importance of ruling out macroprolactinaemia in hyperprolactinaemic subjects, routine screening has not yet been generally adopted. It is particularly striking that none of the recent series of patients with macroprolactinaemia have emanated from the United States (Table 1). It has been projected that approximately 10% of hyperprolactinaemic sera reported in the United States may be accounted for by macroprolactin.23 This estimate is based on the known reactivity of PRL immunoassays and the frequency of their use in the external quality assessment programme of the College of American Pathologists.95 Some authors have argued that macroprolactin levels should only be sought in circumstances where discrepant biochemical and clinical data are found,54 or alternatively where a diagnosis of idiopathic hyperprolactinaemia has been made. Others have suggested that macroprolactin should be measured in patients in whom the dynamic response to PRL stimulating tests is unexpectedly normal. However, the findings in the retrospective study of Suliman et al.23 indicate that differentiation of macroprolactinaemic patients from patients with true hyperprolactinaemia on clinical grounds is unreliable, giving rise to incorrect diagnosis and a missed or delayed opportunity to make the correct diagnosis. Such subjects are likely to undergo unnecessary imaging studies and be prescribed inappropriate pharmacological treatment. Screening for macroprolactin following pituitary imaging or dynamic testing is clearly wasteful of resources and in the case of CT scanning, exposes the patient to unnecessary radiation. In line with others,14,21,51 we recommend routine screening of all hyperprolactinaemic sera for the presence of macroprolactin.
Conclusions
In the majority of clinical situations, macroprolactin results from the binding of monomeric PRL to an endogenous anti-PRL autoantibody. In this high molecular mass complexed form, PRL is rendered bio-unavailable and consequently appears bio-inactive in vivo. Because of reduced metabolic clearance, macroprolactin accumulates in serum leading to hyperprolactinaemia, which is identified by all commercial PRL immunoassays examined. Correct diagnosis of true hyperprolactinaemia is of clinical importance as it is associated with significant morbidity, which is amenable to highly successful treatment. By contrast, misdiagnosis of hyperprolactinaemia due to the presence of macroprolactin leads to patient mismanagement. This may involve inappropriate imaging investigations and treatment, either medical or surgical. Furthermore, identification of the true cause of symptoms will at least be delayed and may lead to missed opportunities to conceive for those patients presenting with infertility. It is conservatively estimated that 10% of biochemical hyperprolactinaemia reported is misleading because of the presence of macroprolactin.23 The distinction between true hyperprolactinaemia and macroprolactinaemia cannot reliably be made on the basis of clinical presentation alone. As a consequence, screening for macroprolactin must be included in the routine investigation of all hyperprolactinaemic patients. Diagnostic companies should be encouraged to develop PRL assays capable of accurately and specifically quantifying bioactive serum monomeric PRL rather than total PRL, as is the case at the moment. Finally, further studies are warranted to address the aetiology of macroprolactin and the clinical implication of the underlying autoimmunity in affected patients.




