British Society for Haematology Guidelines on transfusion for fetuses, neonates and older children (Br J Haematol. 2016;175:784–828). Addendum August 2020
Following publication of the British Society for Haematology (BSH) transfusion guidelines for fetuses, neonates and older children,1 there have been a number of changes requiring this addendum, prior to full revision of the guideline. For each update in the addendum, the relevant section number in the main guideline is given.
Neonatal platelet transfusions (Section 2.3 and Appendix 1, Table c)
The PlaNeT-2/MATISSE study was a multicentre randomised controlled trial of prophylactic platelet transfusions in preterm neonates (660 babies, <34 weeks’ gestation). The results showed that babies with severe thrombocytopenia randomised to receive platelet transfusions to maintain a platelet count threshold of 50 × 109/l had a significantly higher rate of mortality or major bleeding within 28 days of randomisation than those transfused to maintain a platelet count threshold of 25 × 109/l.2 The possible reasons for this evidence of harm of platelet transfusions are not clear and are the subject of further investigation.3 The PlaNeT-2/MATISSE results support strengthening the current BSH guideline restrictive recommendation on prophylactic platelet transfusion thresholds.
Strengthened recommendation on neonatal platelet transfusion thresholds
New additional wording is shown in italics:
For preterm neonates with very severe thrombocytopenia (platelet count below 25 × 109/l) platelet transfusions should be administered in addition to treating the underlying cause of the thrombocytopenia (2C). For non-bleeding neonates platelet transfusions should not be routinely administered if platelet count is ≥25 × 109/l (1B). Suggested threshold counts for platelet transfusions in different situations are given in Table 2 (2C).
New neonatal/infant specification platelet component in plasma/platelet additive solution
- From January 2020, ‘neonatal’ platelet packs manufactured by NHS Blood and Transplant (NHSBT; ~50 ml in plasma), suitable for neonatal and infant transfusion, now contain an additional small volume (~13 ml/pack, 20% by volume) of platelet additive solution (PAS). This is to improve pH and other platelet quality measures. Other UK blood services may also make this change, but in the meantime continue to provide neonatal platelets in plasma as before.
- The number of platelets transfused for a given volume of this new component of platelets in plasma/PAS will be reduced by ~20%. However, this is considered unlikely to be clinically significant, particularly for prophylactic transfusions to non-bleeding neonates. In settings such as cardiac surgery where neonates may be bleeding and transfusion volume less of a consideration, clinicians may choose to transfuse higher volumes by utilising the additional pack volume resulting from the added PAS.
- Note: neonatal platelet transfusion doses (commonly 10–20 ml/kg) are relatively higher than adult equivalents (~2–4 ml/kg). In view of concern about transfusion-associated circulatory overload it is suggested that neonatologists do not increase the prophylactic platelet transfusion volume to compensate for a possible 20% reduction in dose in non-bleeding neonates.
Implications of the SaBTO 2019 advice to cease recommending imported plasma and use of apheresis platelets for individuals born on or after 1 January 1996 or with thrombotic thrombocytopenia (TTP) (Sections 7 and 9, Table IV and Appendix 1)
The requirement to import plasma for treatment of individuals born on or after 1 January 1996 or with TTP was introduced in 2004 in the UK, as part of variant Creuzfeldt–Jacob disease (vCJD) risk reduction measures. In September 2019, the Department of Health and Social Care withdrew this requirement and approved the use of UK-sourced plasma and pooled platelets for these individuals (https://www.parliament.uk/business/publications/written-questions-answers-statements/written-statement/Commons/2019-09-09/HCWS1821/). This followed publication of advice and a comprehensive updated assessment of the vCJD risk by the Advisory Committee for the Safety of Blood, Tissues and Organs (SaBTO) (https://www.gov.uk/government/publications/risk-reduction-measures-for-variant-creutzfeldt-jakob-disease-pcwg-report). For details on the use of plasma for treatment of patients with TTP also refer to the relevant BSH guideline.4
The Joint UK Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee (JPAC) subsequently agreed that UK plasma for those born on or after 1 January 1996 does not need to be pathogen inactivated, similar to UK plasma for all other age groups (Oct 2019, https://www.transfusionguidelines.org/about/minutes-of-jpac-meetings).
Plasma components for neonates, infants and older children
- For neonates and infants, new specifications for neonatal/infant FFP and cryoprecipitate, including the general requirements for neonatal/infant components, are given in the UK ‘Guidelines for the Blood Transfusion Services’ (https://www.transfusionguidelines.org/red-book/chapter-7-specifications-for-blood-components). The neonatal/infant plasma components will all be negative for high-titre (HT) anti-A and anti-B. Note: neonatal/infant FFP has a maximum 24 h shelf-life post thawing when stored at 4 ± 2°C (in contrast to standard FFP which may be used up to 120 h post thawing at 4 ± 2°C for recipients with unexpected major haemorrhage).
- For children from 1 year of age, FFP and cryoprecipitate will be the standard UK ‘adult’ component. HT-negative ‘adult’ FFP is available but not all components are routinely tested.
- Apart from the change in plasma component specifications, other information on plasma transfusion in the guidelines, e.g. indications or typical transfusion volumes and rates, is essentially unchanged. The recommended blood group selections in Table IV are unchanged, but if non-compatible groups are used in emergencies the plasma should be HT negative.
Platelet components for neonates, infants and older children
- Neonatal platelets – should be used for neonates and infants (see New neonatal/infant specification platelet component in plasma/platelet additive solution above)
- For children from 1 year of age, either standard apheresis or pooled platelets may be used.
Neonatal exchange component reissue within hospitals (Appendix 1, Table b)
- Note: ETUs are usually group O, have more plasma (~100 ml) than standard red cells in saline, adenine, glucose and mannitol (SAGM), and a higher volume (NHSBT mean 361 vs. 280 ml). Reissue for indications other than neonatal exchange transfusion should be to recipients who are blood group identical (usually group O) to reduce the risk of haemolysis, and who are not considered at risk of transfusion-associated circulatory overload.
Red cell transfusion formula clarification (Section 6.1.2)
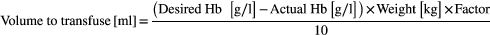
- Note: the Factor is 3–5 (see wording in guideline).
Acknowledgements
Helen V. New and Simon J. Stanworth wrote the initial manuscript, all authors critically reviewed it and approved the final version. We gratefully acknowledge the comments of the BSH Transfusion Task Force and review by paediatric haematology and neonatology expert colleagues.




