Autologous stem cell transplantation for untreated transformed indolent B-cell lymphoma in first remission: an international, multi-centre propensity-score-matched study
Summary
High-dose chemotherapy (HDC) and autologous stem cell transplantation (ASCT) are used as consolidation in first remission (CR1) in some centres for untreated, transformed indolent B-cell lymphoma (Tr-iNHL) but the evidence base is weak. A total of 319 patients with untreated Tr-iNHL meeting prespecified transplant eligibility criteria [age <75, LVEF ≥45%, no severe lung disease, CR by positron emission tomography or computed tomography ≥3 months after at least standard cyclophosphamide, doxorubicin, vincristine and prednisolone with rituximab (R-CHOP) intensity front-line chemotherapy] were retrospectively identified. Non-diffuse large B-cell lymphoma transformations were excluded. About 283 (89%) patients had follicular lymphoma, 30 (9%) marginal-zone lymphoma and six (2%) other subtypes. Forty-nine patients underwent HDC/ASCT in CR1, and a 1:2 propensity-score-matched cohort of 98 patients based on age, stage and high-grade B-cell lymphoma with MYC, BCL2 and/or BCL6 rearrangements (HGBL-DH) was generated. After a median follow-up of 3·7 (range 0·1–18·3) years, ASCT was associated with significantly superior progression-free survival [hazard ratio (HR) 0·51, 0·27–0·98; P = 0·043] with a trend towards inferior overall survival (OS; HR 2·36;0·87–6·42; P = 0·1) due to more deaths from progressive disease (8% vs. 4%). Forty (41%) patients experienced relapse in the non-ASCT cohort — 15 underwent HDC/ASCT with seven (47%) ongoing complete remission (CR); 10 chimeric antigen receptor-modified T-cell (CAR-T) therapy with 6 (60%) ongoing CR; 3 allogeneic SCT with 2 (67%) ongoing CR. Although ASCT in CR1 improves initial duration of disease control in untreated Tr-iNHL, the impact on OS is less clear with effective salvage therapies in this era of CAR-T.
Introduction
Large-cell transformation of indolent B-cell lymphoma (Tr-iNHL) can be manifested at initial presentation or occur over time and accounts for 13% of de novo presentations of diffuse large B-cell lymphoma (DLBCL).1, 2 Several retrospective studies suggest that untreated Tr-iNHL may have similar outcomes to non-transformed DLBCL in the rituximab era.3-6 The optimal treatment approach of untreated Tr-iNHL is unknown, but is generally extrapolated from prospective clinical trials in de novo DLBCL. Additionally, some centres implement high-dose chemotherapy (HDC) and autologous stem cell transplantation (ASCT) as consolidation in first remission (CR1) in fit patients where the transformed disease is not encompassable in a single loco-regional radiation field. This approach is based on the understanding that transformation of low-grade lymphoma has a poor prognosis, but is supported by limited data.1, 7, 8 We performed an international, multi-centre, retrospective study of transplant-eligible patients with untreated Tr-iNHL to assess the impact of consolidation ASCT in CR1 versus no ASCT in CR1 on progression-free survival (PFS) and overall survival (OS). In addition, we assessed the impact of patient and disease factors on outcomes. Finally, we describe patterns of relapse and subsequent salvage therapies to determine the effect of up-front ASCT consolidation on treatment sequencing and outcomes.
Patients and methods
Patients
Transplant-eligible patients with biopsy-proven Tr-iNHL were retrospectively identified across three sites in Australia and the United States diagnosed between January 2000 and January 2019. Cases were reviewed by haematopathologists at each academic medical centre per routine clinical practice. Chronic lymphocytic leukaemia (CLL/SLL), mantle cell lymphoma (MCL) as primary diseases and non- DLBCL transformations were excluded. Composite disease was defined as aggressive and indolent lymphoma diagnosed within the same tissue sample. Sequential transformation was defined as a diagnosis of transformed lymphoma following an antecedent diagnosis of indolent lymphoma. Patients who received prior therapy for indolent lymphoma were excluded. Concordant bone marrow involvement with Tr-iNHL was defined as ≥5% involvement with large-cell lymphoma, and discordant bone marrow involvement defined as morphologic evidence of bone marrow involvement with indolent lymphoma only.9 Flow cytometry and immunohistochemistry studies were utilized to confirm a clonal B-cell population or aberrant immunophenotype where there was unresolved suspicion of involvement by lymphoma. Criteria and methods for performance and interpretation of fluorescence in situ hybridization (FISH) for MYC, BCL2 and BCL6 rearrangements were per the policy of each centre.
Transplant eligibility was defined as: age <75 years; left ventricular ejection fraction (LVEF) ≥45%; no severe lung disease; standard dose rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) chemotherapy or greater intensity front-line chemotherapy; complete remission (CR) by positron emission tomography (PET) or computed tomography (CT) imaging ≥3 months after front-line chemotherapy. Patients with clinical, radiological or histopathologic evidence of relapsed or refractory disease any time after completing front-line chemotherapy were excluded. Patients receiving salvage chemotherapy due to lack of response or refractory disease were excluded. ASCT was performed as consolidation of complete remission achieved after front-line chemotherapy. One Australian centre routinely employed HDC and ASCT in CR1 in transplant-eligible patients, while two did not. Additionally, HDC and ASCT in CR1 were performed at the other two sites at the discretion of the treating physician for patients with high-grade B-cell lymphoma with MYC, BCL2 and/or BCL6 rearrangements (HGBL-DH). This study was approved by the institutional review board of each participating centre.
Statistical analysis
Landmark analysis was performed with time zero at three months after completion of front-line chemotherapy. PFS was defined as the interval from time zero to disease relapse, death from any cause or last follow-up. OS was defined as the interval from time zero to death from any cause or last follow-up. Disease response by CT and/or PET was determined by the Revised Response Criteria for Malignant Lymphoma.10 The chi-square test or Fisher’s exact test was used to evaluate the association between two categorical variables. Wilcoxon rank sum test was used to evaluate the difference in a continuous variable between patient groups. Propensity score matching (PSM) analysis using the ‘greedy match’ algorithm was used to match the baseline covariates to adjust for potential selection bias.11 The Kaplan–Meier method was used for time-to-event analysis including PFS and OS. Univariable (UVA) and multivariable (MVA) Cox proportional hazards models were fitted to the data after PSM to evaluate the association between ASCT in CR1 and time-to-event outcomes. Covariates with P values <0·2 from UVA were used to build the MVA model, and a backward model selection method was applied. The analyses were performed using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria. https://cran.r-project.org; retrieved 2020-02-29) and SAS 9.4 (SAS, Cary, NC, USA).
Results
Patient characteristics
A total of 319 transplant-eligible patients were identified with the following underlying indolent histologies: 283 (89%) patients with follicular lymphoma, 30 (9%) marginal-zone lymphoma, five (2%) mucosa-associated lymphoid tissue (MALT) lymphoma and one (0·3%) Waldenström macroglobulinaemia. About 49 patients underwent HDC and ASCT in CR1. A matched cohort of 98 patients based on age, stage and HGBL-DH at diagnosis was generated with a 1:2 ratio using PSM. The standardized differences of less than 9% for age, sex, stage, Eastern Cooperative Oncology Group Performance Status (ECOG PS) and HGBL-DH suggested that these variables were balanced between the two treatment groups after PSM. The associations between ASCT in CR1 and other covariates were evaluated (Table I). Patient, disease and treatment characteristics were similar between patients treated with ASCT in CR1 versus no ASCT in CR1, with the following exceptions in the ASCT cohort: (i) fewer patients with sequential transformations or having received maintenance rituximab, and (ii) more patients with elevated actate dehydrogenase (LDH).
|
No ASCT (n = 98) |
ASCT (n = 49) |
Total (n = 147) |
P-value | |
|---|---|---|---|---|
| Centre | ||||
| Peter MacCallum Cancer Centre | 0 | 39 (80%) | 39 (27%) | – |
| Sir Charles Gairdner Hospital | 3 (3%) | 1 (2%) | 4 (3%) | |
| UT MD Anderson Cancer Center | 95 (97%) | 9 (18%) | 104 (71%) | |
| *Age (range) | 56 (26–74) | 56 (29–74) | 56 (26–74) | 0·6 |
| *Sex | ||||
| Male | 57 (58%) | 30 (61%) | 87 (59%) | 0·9 |
| Female | 41 (42%) | 19 (39%) | 60 (41%) | |
| *Stage (Ann Arbor) | ||||
| 1 or 2 | 9 (9%) | 5 (10%) | 14 (10%) | 1·0 |
| 3 or 4 | 89 (91%) | 44 (90%) | 133 (90%) | |
| *HGBL-DH | ||||
| Yes | 15 (15%) | 9 (18%) | 24 (16%) | 0·6 |
| No/ not tested | 83 (85%) | 40 (82%) | 123 (84%) | |
| *ECOG PS | ||||
| 0 or 1 | 90 (95%) | 47 (96%) | 137 (95%) | 1·0 |
| ≥2 | 5 (5%) | 2 (4%) |
7 (5%) |
|
| Indolent lymphoma subtype | ||||
| Follicular lymphoma | 91 (93%) | 42 (86%) | 133 (90%) | 1·0 |
| Marginal zone lymphoma | 5 (5%) | 3 (6%) |
8 (5%) |
|
| Waldenström macroglobulinaemia | 0 | 1 (2%) | 1 (1%) | |
| MALT lymphoma | 2 (2%) | 0 | 2 (1%) | |
| Composite lymphoma | ||||
| Yes | 79 (81%) | 34 (69%) | 113 (77%) | 0·2 |
| No | 19 (19%) | 15 (31%) |
34 (23%) |
|
| Timing of transformation | ||||
| At presentation | 84 (86%) | 48 (98%) | 132 (90%) | 0·02 |
| Sequential | 14 (14%) | 1 (2%) |
15 (10%) |
|
| LDH | ||||
| Normal | 53 (67%) | 18 (38%) | 71 (56%) | 0·002 |
| Elevated | 26 (33%) | 30 (62%) | 56 (44%) | |
| Not recorded | 19 | 1 | 20 | |
| Extranodal sites | ||||
| 0 or 1 sites | 65 (68%) | 31 (63%) | 96 (66%) | 0·7 |
| ≥2 sites | 31 (32%) | 18 (37%) | 49 (34%) | |
| Not recorded | 2 | 0 | 2 | |
| IPI | ||||
| IPI 0 or 1 |
21 (27%) |
12 (25%) |
33 (26%) |
0·9 |
| IPI 2 or 3 | 51 (66%) | 32 (67%) | 83 (66%) | |
| IPI 4 or 5 | 5 (6%) | 4 (8%) | 9 (7%) | |
| Not recorded | 21 | 1 | 22 | |
| B symptoms | ||||
| Yes | 26 (27%) | 12 (24%) | 38 (26%) | 0·8 |
| No | 69 (73%) | 37 (76%) | 106 (74%) | |
| Not recorded | 3 | 0 |
3 |
|
| Bulk (≥10 cm) | ||||
| Yes | 12 (13%) | 12 (24%) | 24 (17%) | 0·1 |
| No | 81 (87%) | 37 (76%) | 118 (83%) | |
| Not recorded | 5 | 0 |
5 |
|
| Type of BM involvement | ||||
| Discordant | 23 (25%) | 20 (42%) | 43 (31%) | 0·6 |
| Concordant | 13 (14%) | 8 (16%) | 21 (15%) | |
| Not involved | 55 (60%) | 20 (42%) | 75 (54%) | |
| Not assessed | 7 | 1 | 8 | |
| Front-line chemotherapy | ||||
| R-CHOP | 74 (76%) | 36 (73%) | 110 (75%) | 0·6 |
| Da-EPOCH-R | 20 (20%) | 10 (20%) | 30 (20%) | |
| RHCVAD | 2 (2%) | 3 (6%) | 3 (6%) | |
| O-CHOP + lenalidomide | 2 (2%) | 0 | 0 | |
| Conditioning chemotherapy | ||||
| BEAM | – | 10 (20%) | 10 (20%) | – |
| Cyclophosphamide, carmustine, etoposide |
– |
36 (73%) |
36 (73%) |
|
| Busulfan, melphalan | – | 1 (2%) | 1 (2%) | |
| Busulfan, melphalan, gemcitabine, SAHA | – | 2 (4%) | 2 (4%) | |
| Maintenance rituximab | ||||
| No | 77 (79%) | 45 (92%) | 122 (83%) | 0·06 |
| Yes | 21 (21%) | 4 (8%) |
25 (17%) |
|
| Disease status at first relapse or last follow-up | ||||
| Indolent lymphoma | 14 (14%) | 2 (4%) | 16 (11%) | 0·5 |
| Large cell/transformed lymphoma | 26 (27%) | 9 (18%) | 35 (24%) | |
| Remission | 58 (59%) | 38 (78%) | 96 (65%) |
- PSM, Propensity score matching; ASCT, Autologous stem cell transplantation; HGBL-DH, High-grade B-cell lymphoma with MYC, BCL2 and/or BCL6 rearrangements; ECOG, Eastern Cooperative Oncology Group; PS, performance status; MALT, mucosa-associated lymphoid tissue; LDH, lactate dehydrogenase; IPI, International Prognostic Index; BM, bone marrow; R-CHOP, Rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; DA-EPOCH-R, Dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, rituximab; RHCVAD, rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone alternating with methotrexate and cytarabine; O-CHOP, Obinutuzumab, cyclophosphamide, doxorubicin, vincristine, prednisone; BEAM, carmustine, etoposide, cytarabine, melphalan; SAHA, suberoylanilide hydroxamic acid.
- * 1:2 ratio PSM performed using based on age, Ann Arbor stage, HGBL-DH status.
Outcomes
Reported outcomes and survival analysis were performed on the PSM cohort (n = 147). With a median follow-up of 3·7 (range 0·1–18·3) years (estimated from the censored observations), the four-year OS and PFS rates were 91% [95% confidence interval (CI): 0·86–0·97] and 61% (95% CI: 0·527–0·706) respectively. The median follow-up was similar in patients receiving ASCT in CR1 (3·7 years; range 0·2–10·6) compared to patients who did not receive ASCT in CR1 (3·5 years; range 0·1–18·3).
By univariable analysis, no significant differences in four-year PFS or OS rates were observed with follicular lymphoma (FL) versus non-FL indolent histology (PFS: 66% vs. 61%; P = 0·7; OS: 91% vs. 90%; P = 0·2), sequential versus non-sequential transformation (PFS: 75% vs. 65%; P = 0·4; OS: 91% vs. 91%; P = 0·9) or advanced [3 or 4] vs. limited stage [1 or 2] disease (PFS: 66% vs. 67%; P = 0·4; OS: 90% vs. 100%; P = 0·7). The factors associated with inferior PFS and OS by UVA were HGBL-DH (PFS: HR 3·0, 95% CI 1·5–5·8, P = 0·001; four-year PFS 39% vs. 70%; OS: HR 5·1, 95% CI 1·5–17·2, P = 0·009; four-year OS 79% vs. 93%) and ‘high’ International Prognostic Index (IPI) score [4 or 5] (PFS: HR 3·7, 95% CI 1·4–9·5, P = 0·007; four-year PFS 41% vs. 75%; OS: HR 8·1, 95% CI 2·1–30·8, P = 0·002; four-year OS 60% vs. 96%) (Table II). The following factors were associated with inferior PFS without an impact on OS: B symptoms (PFS: HR 2·5, 95% CI 1·4–4·4, P = 0·001; four-year PFS 45% vs. 75%; four-year OS 89% vs. 93%, P = 0·5) and ≥2 extranodal sites (PFS: HR 2·1, 95% CI 1·2–3·7, P = 0·006; four-year PFS 53% vs. 73%; four-year OS 89% vs. 92%, P = 0·8). A trend towards inferior PFS was observed with concordant versus no bone marrow (BM) involvement (HR 1·7, 95% CI 0·8–3·9, P = 0·18; four-year PFS 63% vs. 71%), discordant versus no BM involvement (HR 1·8, 95% CI 1·0–3·2, P = 0·05; four-year PFS 62% vs. 71%) and Eastern Cooperative Oncology Group performance status (ECOG PS) >1 vs. ECOG PS 0 or 1 (HR 2·0, 95% CI 0·7–5·6, P = 0·2; four-year PFS 54% vs. 67%). A trend towards improved survival outcomes was observed with composite lymphoma versus non-composite presentations (PFS: HR 0·6, 95% CI 0·3–1·1, P = 0·1; four-year PFS 69% vs. 56%; OS: HR 0·4, 95% CI 0·1–1·3, P = 0·1; four-year OS 92% vs. 87%), maintenance rituximab (PFS: HR 0·6, 95% CI 0·3–1·3, P = 0·2; four-year PFS 79% vs. 63%; OS: HR 0·2, 95% CI 0·1–1·6, P = 0·1; four-year OS 100% vs. 89%) and ASCT in CR1 (PFS: HR 0·6, 95% CI 0·3–1·0, P = 0·07; four-year PFS 77% vs. 60%; OS: HR 2·4, 95% CI 0·9–6·4, P = 0·09; four-year OS 88% vs. 93%) (Fig 1).
|
PFS analysis |
OS analysis | ||||||
|---|---|---|---|---|---|---|---|
|
HR |
95% CI |
P value |
HR |
95% CI |
P value | ||
|
Age >60 years |
1·1 |
0·6–2·0 |
0·8 |
1·1 |
0·4–3·2 |
0·8 |
|
|
Male gender |
1·0 |
0·6–1·8 |
0·9 |
0·9 |
0·4–2·5 |
0·9 |
|
|
Composite vs. non-composite transformation |
0·6 |
0·3–1·1 |
0·1 |
0·4 |
0·1–1·3 |
0·1 |
|
|
Sequential vs. non-sequential transformation |
0·6 |
0·2–1·9 |
0·4 |
0·9 |
0·1–7·0 |
0·9 |
|
|
Indolent lymphoma histology — FL vs. non-FL histology |
1·2 |
0·5–2·9 |
0·7 |
2·1 |
0·6–7·6 |
0·2 |
|
| Aggressive lymphoma histology — HGBL-DH vs. non-HGBL-DH/not tested |
3·0 |
1·5–5·8 |
0·001 |
5·1 |
1·5–17·2 |
0·009 |
|
|
IPI “high” (4/5) vs. IPI “low/intermediate” (0–3) |
3·7 |
1·4–9·5 |
0·007 |
8·1 |
2·1–30·8 |
0·002 |
|
|
Advanced stage (3/4) vs. limited stage (1/2) |
1·5 |
0·6–4·3 |
0·4 |
1·5 |
0·2–11·6 |
0·7 |
|
|
ECOG PS ≥2 |
2·0 |
0·7–5·6 |
0·2 |
3·9 |
0·9–17·4 |
0·08 |
|
|
Elevated LDH |
1·2 |
0·6–2·3 |
0·6 |
2·1 |
0·7–6·7 |
0·2 |
|
|
Extranodal sites ≥2 |
2·1 |
1·2–3·7 |
0·006 |
1·1 |
0·4–3·0 |
0·8 |
|
|
Presence of B symptoms |
2·5 |
1·4–4·4 |
0·001 |
1·5 |
0·5–4·3 |
0·5 |
|
|
Bulk ≥10 cm |
0·7 |
0·3–1·7 |
0·5 |
0·7 |
0·2–3·1 |
0·6 |
|
|
BM involvement vs. not involved (overall) |
– |
– |
0·12 |
– |
– |
0·7 |
|
| Concordant BM involvement vs. not involved | 1·9 | 0·8–4·1 | 0·14 | 1·7 | 0·5–6·5 | 0·4 | |
|
Discordant BM involvement vs. not involved |
1·7 |
1·0–3·2 |
0·07 |
1·2 |
0·4–3·4 |
0·8 |
|
|
Up-front chemotherapy with R-CHOP vs. DA-EPOCH-R |
0·7 |
0·3–1·6 |
0·5 |
0·6 |
0·1–4·4 |
0·6 |
|
|
Maintenance rituximab vs. no maintenance |
0·6 |
0·3–1·3 |
0·2 |
0·2 |
0·02–1·41 |
0·1 |
|
|
ASCT in CR1 vs. no ASCT |
0·6 | 0·3–1·0 | 0·07 | 2·4 | 0·9–6·4 | 0·09 | |
- PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; FL, follicular lymphoma; HGBL-DH, high-grade B-cell lymphoma with MYC, BCL2 and/or BCL6 rearrangements; IPI, International Prognostic Index; ECOG, Eastern Cooperative Oncology Group; PS, performance status; LDH, lactate dehydrogenase; BM, bone marrow; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; DA-EPOCH-R, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, rituximab; ASCT, autologous stem cell transplantation; CR1, first remission.
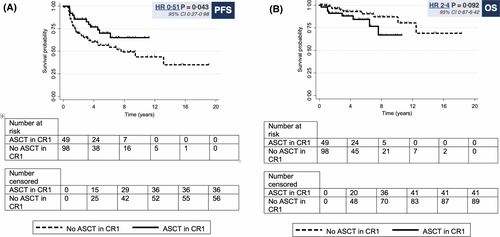
By MVA, ASCT in CR1 was independently associated with improved PFS (HR 0·5, 95% CI 0·27–0·98; P = 0·043) (Table III) with the adjustment of the other important covariates in the model. The factors associated with inferior PFS were HGBL-DH (HR 2·31, 95% CI 1·10–4·84; P = 0·026) and B symptoms (HR 1·9, 95% CI 1·1–3·5; P = 0·03). The overall P-value for the association between BM involvement versus not involved was 0·104 (discordant BM involvement versus not involved [HR 1·9, 95% CI 1·03–3·60; P = 0·04]; concordant BM involvement versus not involved [HR 1·72, 95% CI 0·75–3·94; P = 0.2]). Due to a limited number of deaths, MVA was not performed for OS analysis.
|
PFS |
|||
|---|---|---|---|
|
HR |
95% CI | P value | |
|
ASCT in CR1 vs. no ASCT |
0·51 |
0·27–0·98 |
0·043 |
|
HGBL-DH vs. non-HGBL-DH or not tested |
2·31 |
1·10–4·84 |
0·026 |
|
B symptoms vs. no B symptoms |
1·92 |
1·05–3·50 |
0·034 |
|
Concordant BM involvement vs. not involved |
1·72 |
0·75–3·94 |
0·200 |
|
Discordant BM involvement vs. not involved |
1·93 | 1·03–3·60 | 0·040 |
- PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; ASCT, autologous stem cell transplantation; CR1, first remission; HGBL-DH, high-grade B-cell lymphoma with MYC, BCL2 and/or BCL6 rearrangements; BM, bone marrow.
Relapse and deaths
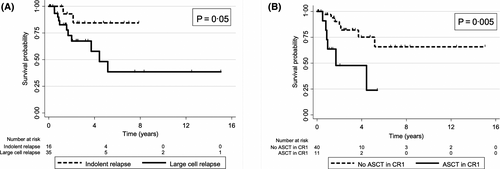
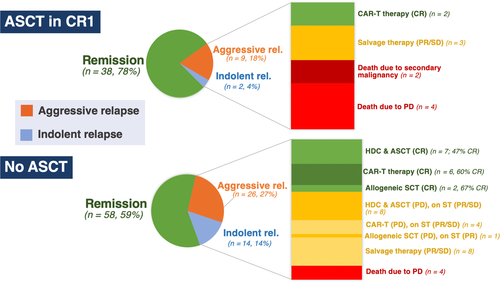
Fifty-one patients experienced lymphoma relapse with an estimated four-year post-relapse survival rate of 68%. Sixteen (31%) patients experienced relapse with indolent lymphoma only (median PFS 1·4 years, four-year postrelapse OS 87%) and 35 (69%) with large-cell or transformed lymphoma (median PFS 1·4 years, four-year postrelapse OS 74%, Fig 2A). Fewer relapses occurred among patients who underwent ASCT in CR1 vs. no ASCT in CR1 (22% vs. 41%) due to fewer relapses with indolent lymphoma (4% vs. 14%) (Fig 3, and Table S1). Eleven (22%) patients in the ASCT in CR1 cohort experienced relapse (median PFS 1·4 years, four-year OS 73%). At last follow-up, four (36%) had died due to progressive disease (PD), two (18%) died due to secondary malignancy, two (18%) underwent chimeric antigen receptor-modified T-cell (CAR-T) therapy with ongoing CR, three (27%) continue on salvage therapy. About 40 (41%) patients in the no ASCT in CR1 cohort experienced relapse (median PFS 1·2 years, four-year OS 84%, Fig 2B) — at last follow-up, four (10%) died due to PD, 15 underwent HDC and ASCT with 7/15 [47%] ongoing CR; 10 underwent CAR-T therapy (five relapse post-ASCT, four refractory disease, one relapse post-CR1) with 6/10 [60%] ongoing CR; three underwent allogeneic SCT (two relapse post-ASCT, one relapse post-CR1) with 2/3 [67%] ongoing CR. The remaining patients experienced relapse with either iNHL or aggressive (a)-NHL and continue on salvage therapy or investigational agents with ongoing response. There were 17 deaths across the entire cohort. The four-year non-relapse mortality (NRM) rate for the entire cohort was 6%, with no significant difference between patients treated with ASCT in CR1 (9%) versus no ASCT in CR1 (5%), P = 0·5. Causes of NRM in the ASCT in CR1 cohort include: one (2%) due to sepsis, one (2%) Parkinson’s disease, two (4%) secondary malignancy. Causes of NRM in the no ASCT in CR1 cohort include: one (1%) due to treatment-related toxicity, one (1%) secondary malignancy, three (3%) unknown causes unrelated to lymphoma.
Discussion
In this international, multi-centre retrospective analysis of untreated Tr-iNHL patients in the rituximab era, we observed improved initial duration of disease control with up-front ASCT in CR1 largely due to fewer relapses with indolent disease. In addition, NRM was not significantly increased in the ASCT in CR1 cohort (9% vs. 5%, P = 0·5) and there was no excess of secondary malignancies. Our findings are consistent with a retrospective analysis by the Canadian group of 105 patients with Tr-iNHL, 82% having received prior therapy of whom 50 patients proceeded with ASCT consolidation. Patients who underwent ASCT in the rituximab era demonstrated favourable outcomes with a three-year PFS of 54% and OS of 69%.12 Similar findings have been demonstrated in a single-centre, retrospective Norwegian study.13 In a separate retrospective study, investigators at Washington University School of Medicine reported on 105 patients with transformed FL of whom 24 underwent ASCT as consolidation therapy. Although survival outcomes were superior in the ASCT versus non-ASCT cohorts (five-year PFS rate: 42% vs. 30%; five -year OS rate: 74% vs. 63%) this did not reach statistical significance.14 Of note, the ASCT cohort was heavily pretreated with 62% of patients receiving prior therapy for FL compared to 38% in the non-ASCT cohort, possibly accounting for this difference. A retrospective Danish study of 85 patients with Tr-iNHL of whom 54 received ASCT consolidation reported a PFS improvement in sequential transformations, but not composite/discordant Tr-iNHL. In this context, 76% of sequential transformations had received one or more therapies prior to transformation thereby being more representative of a salvage ASCT rather than consolidation ASCT in CR1.15 There were too few patients with sequential transformations who received ASCT in CR1 in our study for meaningful interpretation. By limiting our analysis to treatment-naïve patients and using PSM to reduce bias, our study provides robust evidence examining the impact of ASCT in CR1 in patients with Tr-iNHL. However, small numbers and a relatively short follow-up of 3·7 years may limit the interpretation of these findings. With an estimated number needed to treat (NNT) of six to prevent a single progression event with up-front ASCT, the true survival benefit needs to be weighed carefully against the short- and long-term complications of high-dose chemotherapy (HDC), financial costs and excellent survival outcomes at relapse, especially with indolent lymphoma.
The presence of MYC rearrangements in de novo DLBCL is associated with inferior survival outcomes.16, 17 In our study, HGBL-DH was identified in 16% of Tr-iNHL and was independently associated with inferior survival outcomes. A retrospective study of 312 patients with newly diagnosed DLBCL of whom 63 had concurrent or antecedent indolent lymphoma demonstrated similar findings of inferior survival outcomes associated with HGBL-DH. Despite HGBL-DH demonstrating equivalently poor outcomes in transformed and non-transformed DLBCL, a higher rate of HGBL-DH was reported in the transformed versus non-transformed DLBCL cohorts (32% vs. 11%).3 This may be explained by the high prevalence of BCL2 translocations in FL; however, lack of systematic MYC FISH testing and selection bias associated with retrospective analysis limits this finding, and needs confirmation in a prospective manner.
Discordant, not concordant BM involvement with transformed lymphoma was independently associated with inferior PFS in our study, but any interpretation is limited by small numbers. Our findings are discrepant to a retrospective analysis of 795 patients of Tr-iNHL which demonstrated concordant BM involvement as an independent negative prognostic marker.18 More than half of patients who presented with concordant BM involvement progressed within the first year with a dismal three-year PFS of 37% and OS of 49%. Therefore, most patients presenting with concordant BM involvement were unlikely to have achieved adequate disease control or survived to be eligible for ASCT in CR1, thereby being excluded from our study and explaining this discrepant finding.
In our study, approximately one third of all relapses occur with indolent disease and the remainder with transformed lymphoma. Patients who underwent ASCT in CR1 experienced fewer relapses largely due to fewer indolent relapses. However, patients in the ASCT in CR1 cohort whose disease relapsed as large-cell lymphoma had inferior overall survival due to poorer responses to second-line and later therapy. This may be accounted for by geographical differences in the availability of potentially curative CAR-T therapy which remains inaccessible in Australia resulting in more progression-related deaths in the ASCT in CR1 cohort. Therefore, the observed clinical benefit of ASCT in CR1 is the prevention of indolent disease relapse. Given the rapidly growing treatment options for indolent B-cell lymphomas, including immunomodulatory agents (lenalidomide), monoclonal antibodies (obinutuzumab), PI3K inhibitors (idelalisib, duvelisib and copanlisib) and other investigational agents (tazemetostat, ibrutinib and venetoclax), the need for ASCT in CR1 is put to question.19-25 Sequencing ASCT as second-line or later salvage therapy may spare patients exposure to a high-cost, potentially toxic therapy and associated long-term sequelae. The question of whether ASCT may be potentially curative by preventing indolent disease relapse exists; however, longer follow-up is required. Further studies are needed to identify whether a subset of patients may benefit from potentially curative, up-front ASCT in CR1 such as mutations in TP53, CDKN2A/B, NOTCH1 and MYC.26-28
Our study has limitations. We selected PSM to reduce treatment assignment bias by accounting for multiple baseline covariates by individual matching to patients in the ASCT in CR1 cohort. Despite the efforts made to reduce potential bias, we acknowledge that confounding may exist due to unmeasured patient factors such as co-morbidities and fitness, as well as prespecified practices at each centre determining whether ASCT consolidation was administered. This limitation is inherent to the retrospective nature of our study and limitations of recorded data available which we have attempted to account for by determining prespecified transplant eligibility criteria in the study inclusion criteria. Thirdly, although a trend towards inferior OS was demonstrated, this observation is limited by short follow-up, limited number of events and cross-over effect of patients receiving salvage ASCT at relapse.
Conclusion
Up-front ASCT in CR1 for patients with untreated Tr-iNHL in the rituximab era is associated with improved initial duration of disease control due to fewer relapses with indolent lymphoma. The lack of an OS benefit is likely due to highly effective salvage therapies which may achieve long-term disease control. Further observational and translational studies are needed to identify which patient subgroups may benefit from potentially curative front-line therapy with ASCT in CR1.
Acknowledgements
The authors acknowledge the contributions of Mansoor Noorani (UT MD Anderson Cancer Center) and Kerrie Stokes (Peter MacCallum Cancer Centre) to this study. Portions of this article were presented at the 2020 ASCO Annual Meeting, May 29–31, 2020, Chicago, IL, United States.
Funding information
This work was supported in part by the Cancer Center Support Grant (CCSG) at UT MD Anderson Cancer Center.
Author contributions
CKC and MD: designed the study. CYC, JFS, DR, KB, CST, NF, LEF, JW, SSN, FBH, FS, LN and MD: provided study materials and patients. CKC, KJL, KLL and PJ: collected and assembled the data. CKC, YQ and LF: analysed and interpreted the data. All authors: wrote the manuscript. All authors gave final approval for the manuscript.
Conflicts of interest
CKC, KJL, PJ, YQ, LF, KB, LEF, FS, LEF and FBH: no conflict of interest. KLL: has received honoraria from Roche; has received travel expenses from Janssen, Novartis. CYC: has received consulting/advisory/honoraria from Roche, Janssen, MSD, Gilead, Ascentage Pharma, Acerta, Loxo Oncology, TG Therapeutics; has received research funding from Celgene, Roche, AbbVie; has received travel expenses from Roche. JFS: has received research support from AbbVie, Celgene, Janssen and Roche; has served as consultant and advisory board member for AbbVie, Acerta Pharma, Janssen, Roche, Sunesis Pharmaceuticals and Takeda; Speaker’s Bureau for AbbVie, Celgene and Roche; has received honoraria from AbbVie, Acerta Pharma, Janssen, Roche, Sunesis Pharmaceuticals and Takeda; has received travel expenses from AbbVie and Roche. DR: has received research support from Amgen, Bristol-Myers Squibb, Takeda, Beigene and Imago; has served as consultant and advisory board member for Amgen and Pfizer; has received honoraria from Amgen, Novartis and Sanofi. CT: has received research support from Janssen-Cilag and AbbVie; has served as consultant and advisory board member for Janssen, Loxo, Roche, BeiGene and AbbVie; has received honoraria from Janssen-Cilag, AbbVie, Novartis, Beigene and Pharmacyclics. NF: has received research support from Celgene, Roche, Janssen, TG Therapeutics and AbbVie; has served as consultant and advisory board member for Celgene, Roche, Janssen, TG Therapeutics and AbbVie. JW: has received research support from Novartis, Celgene, Janssen, Kite/Gilead, Unum, Genentech, Curis and 47 Inc; has served as consultant and advisory board member for Novartis, Celgene, Juno, Janssen, Kite/Gilead, MorphoSys, Genentech and Curis. SSN: has received research support from Kite/Gilead, Cellectis, Poseida, Merck, Acerta, Karus, BMS, Unum Therapeutics, Allogene, and Precision Biosciences; has served as consultant and advisory board member for Kite/Gilead, Celgene, Novartis, Unum Therapeutics, Pfizer, Merck, Precision Biosciences, Cell Medica, Incyte, Allogene, Calibr, and Legend Biotech; has patents related to cell therapy. CF: has received research support from AbbVie, Acerta Pharma, Celgene, Gilead Sciences, Infinity Pharmaceuticals, Janssen Pharmaceuticals, Millennium/Takeda, National Institutes of Health, Onyx Pharmaceuticals, Pharmacyclics and Spectrum Pharmaceuticals; has served as consultant and advisory board member for Spectrum Pharmaceuticals, Celgene, Optum Rx, Seattle Genetics, Kite/Gilead and Bayer. LN: has received research support from TG Therapeutics, Janssen, Genentech and Celgene; has served as consultant and advisory board member for TG Therapeutics, Novartis, Janssen, Spectrum Pharmaceuticals, Kite/Gilead, Genentech, Bayer and Celgene. MD: has received research support from Celgene, GSK, Takeda and Novartis; has served as consultant or advisory board member for Roche, GSK, Janssen and Takeda. Celgene, Gilead Sciences and Novartis; has served as a speaker for Roche, Novartis, Janssen, Takeda and Gilead Sciences.




