Updates of incretin-related drugs for the treatment of type 2 diabetes
Graphical Abstract
Because the number of patients with type 2 diabetes is increasing, antidiabetic drugs that are effective and have little risk of causing hypoglycemia have been required over recent decades, especially in aged societies.
Dipeptidyl peptidase-4 (DPP-4) inhibitors have been widely used, especially in East Asia, and are the most used antidiabetic drugs in Japan because of their safety and no negative impact on bodyweight1, 2. In addition to their safety, several reports have shown the efficiency of the drugs in individuals of East Asian origin3-5. A recent report from Korea also showed that the addition of teneligliptin to patients with type 2 diabetes who were inadequately controlled by oral triple combination therapy (metformin, sulphonylureas [SUs] and sodium–glucose cotransporter 2 inhibitors) significantly decreased glycated hemoglobin (HbA1c) levels compared with a placebo (intergroup difference was −0.75%) without increasing adverse events (AEs)6.
Although the risk is not so high, bullous pemphigoid is one of the AEs with DPP-4 inhibitors that we have to be aware of. Recent reports showed that the risk was increased 3 months from the first use of DPP-4 inhibitors7, and Lee et al.8 showed that age, race and the class of the drug are related to a higher risk of bullous pemphigoid. A current study also reported that the administration of DPP-4 inhibitors might be associated with Graves' disease exacerbation; therefore, we have to pay attention when we use the drug in patients with Graves' disease9.
Besides the safety and efficacy of DPP-4 inhibitors, meta-analysis of randomized trials showed that glucagon-like peptide-1 receptor agonists (GLP-1 RAs) reduced the risk of major adverse cardiac events, all-cause mortality, hospital admission for heart failure and the composite kidney outcome, without an increase of adverse effects10. In addition to the protective effects on cardiovascular diseases and kidney function11, GLP-1 RAs can reduce bodyweight12.
GLP-1 RAs lower glucose levels by direct and indirect actions on several organs, including the central nervous system, heart, arteries, kidneys, liver and pancreas13. A recent systematic review and meta-analysis14 also showed that GLP-1RAs significantly reduce both visceral adipose tissue and subcutaneous adipose tissue, and the decrease of visceral adipose tissue was greater than that of subcutaneous adipose tissue when compared with the control group.
GLP-1RAs are an attractive alternative treatment for bolus insulin injections because of their lower risk of hypoglycemia and weight loss benefit, especially for obese patients with type 2 diabetes. A recent report showed that simplification from an intensive insulin injection regimen (basal + bolus insulin injection) to liraglutide maintained the HbA1c level with stable glucose variability in patients with well-controlled type 2 diabetes receiving multiple daily insulin injection therapy15. Furthermore, the replacement of the therapy decreased the total insulin dose and quality of life score, which are significantly associated with injection frequency.
For some older patients with diabetes, even once daily injection is still difficult. Under such a situation, the use of weekly GLP-1 RAs has been approved in Japan. Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) trials elucidated that injection of once-weekly semaglutide achieved better glycemic control, greater reduction of bodyweight and waist circumference, and a lower rate of major adverse cardiac events without increasing serious adverse effects than a placebo or comparators16. Type 2 diabetes patients in East Asian countries are characterized by lower body mass index (BMI) arising from β-cell dysfunction and relatively low insulin secretion capacity compared with those of white people. Age is also an important issue when deciding the treatment of diabetes in a ‘super-aged’ society, such as Japan. From these points of view, SUSTAIN Japan monotherapy and SUSTAIN Japan oral antidiabetes drug assessed the efficacy and safety of semaglutide versus comparators (sitagliptin in the monotherapy study, SUs, glinides, α-glucosidase inhibitors or thiazolidinedione) in Japanese type 2 diabetes patients across subgroups defined by baseline age and BMI17. The study showed both 0.5 mg or 1.0 mg semaglutide administration induced greater reduction of HbA1c and bodyweight in all age subgroups (<65 years or ≥65 years), all BMI subgroups (BMI <25 kg/m2 or ≥25 kg/m2), and all age and BMI subgroups (combination of the former two groups). Although gastrointestinal AEs were more common with semaglutide than comparators, AE rates were broadly similar in all age and BMI subgroups, and no severe or blood glucose-confirmed symptomatic hypoglycemic episodes were reported with semaglutide 0.5 mg or a comparator (only one with semaglutide 1.0 mg).
In addition to weekly subcutaneous semaglutide, the safety and efficacy of oral semaglutide were investigated in global trials and also in two Japanese trials (Peptide Innovation for Early Diabetes Treatment [PIONEER] 9 and 10)18, 19, and oral semaglutide was recently approved in Japan for the treatment of type 2 diabetes. PIONEER 9 showed that 26 (and also 52) weeks of oral semaglutide (3, 7 or 14 mg) significantly reduced HbA1c compared with a placebo in a dose-dependent manner, and at the 14-mg dose, it also showed a significant reduction of HbA1c compared with once-daily liraglutide 0.9 mg. PIONEER 10, evaluating the safety and efficacy of oral semaglutide versus dulaglutide once-weekly injection in patients with type 2 diabetes who were receiving oral antidiabetic monotherapy (SUs, glinides, thiazolidinedione, α-glucosidase inhibitors or sodium–glucose cotransporter 2 inhibitors), showed similar rates of AEs in the oral semaglutide and dulaglutide groups. The study also showed that semaglutide at the 14-mg dose reduced HbA1c, and the 7- and 14-mg doses significantly reduced bodyweight, compared with 0.75 mg dulaglutide. The reduction of HbA1c in these two trials was greater than those in other PIONEER trials in which most of the participants were white. A subgroup analysis of these two trails also elucidated that higher baseline HbA1c (≤8.0, >8.0–≤9.0 or >9.0%) related to higher HbA1c reduction, whereas BMI (<25, ≥25–<30 or ≥30 kg/m2) and background medication showed no interaction20. For AEs, there was no apparent difference between subgroups.
Recently, several clinical trials of tirzepatide, a dual agonist of GLP-1R and glucose-dependent insulinotropic polypeptide receptor have been reported. The SURPASS-2 trial, an open-label, 40-week, phase III trial, demonstrated that all three doses (5,10 or 15 mg) of tirzepatide lowered HbA1c and reduced bodyweight significantly greater than 1 mg of semaglutide, without increasing AEs21. The SURPASS J-mono study enrolled 636 Japanese patients with type 2 diabetes, and compared the safety and efficacy of three doses (5,10 or 15 mg) of tirzepatide with 0.75 mg of dulaglutide for 52 weeks. The reduction of HbA1c was superior in all doses of tirzepatide (−2.37 ~ −2.82%) when compared with dulaglutide (−1.29%), and the reduction of bodyweight was also superior in all groups of tirzepatide (−5.8 ~ −10.7 kg vs −0.5 kg). The most common AEs observed with tirzepatide were gastrointestinal, which were comparable with dulaglutide22. The safety and efficacy of tirzepatide have also been reported in SURPASSJ-combo, in which tirzepatide was added on to single oral antihyperglycemic medication (SUs, biguanides, α-glucosidase inhibitors, thiazolidinedione, glinides or sodium–glucose cotransporter 2 inhibitors) in Japanese patients with type 2 diabetes23.
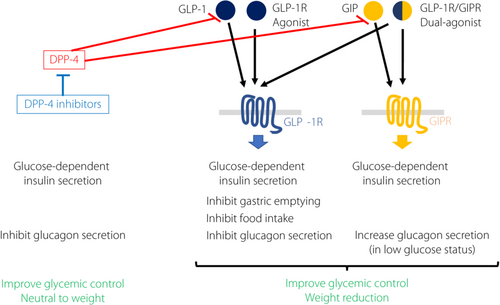
Among incretin-related drugs, because the DPP-4 inhibitors are effective to reduce HbA1c with little risk of hypoglycemia and are neutral to the bodyweight, they might be preferred for older patients with type 2 diabetes. In contrast, GLP-1RAs show greater efficacy in HbA1c reduction and significant reduction in bodyweight. They also provide the patients with multiple choices of administration, such as once or twice-day injection, once-weekly injection and oral administration. Furthermore, they might have protective benefits for atherosclerotic diseases. Therefore, they can be used for obese patients with diabetes and high risk of cardiovascular diseases. Tirzepatide might have greater effects in HbA1c and bodyweight reductions than GLP-1RAs (Figure 1). Therefore, it can also be used for obese patients with type 2 diabetes, but it lacks the evidence for the benefits for cardiovascular diseases at this point.

Disclosure
EA has participated on advisory panels for Abbott Japan, AstraZeneca, Novartis Pharma, Novo Nordisk Pharma, Ono Pharmaceutical, Takeda Pharmaceutical and Terumo Corporation; has received scholarship grants from Astellas Pharma, Bayer, Daiichi Sankyo Company, Eli Lilly Japan, Kowa Company, Kyowa Kirin Co., Mitsubishi Tanabe Pharma, MSD, Nippon Boehringer Ingelheim, Novartis Pharma, Novo Nordisk Pharma, Sanofi, Shionogi, Sumitomo Dainippon Pharma, Taisho Pharmaceutical and Takeda Pharmaceutical; and speaker's fees from Abbott Japan, Arkray, Astellas Pharma, AstraZeneca, Daiichi Sankyo Company, Eli Lilly Japan, Kowa Company, Kyowa Kirin Co., Mitsubishi Tanabe Pharma, MSD, Novartis Pharma, Novo Nordisk Pharma, Ono Pharmaceutical, Sanofi, and Sumitomo Dainippon Pharma, Sanwa Kagaku Kenkyusho Co., Taisho Pharmaceutical, Takeda Pharmaceutical, Terumo Corporation and WebMD. TM has received speaker's fees from Eli Lily Japan KK and Boehringer Ingelheim Japan Inc; and a research grant from Shimazu Corporation. The other authors declare no conflict of interest.